Overview of the Global Anemia Problem, Including Iron Deficiency Anemia
The World Health Organization (WHO) defines anemia among women of childbearing age as the condition of having a hemoglobin concentration of < 12.0 g/dL at sea level; among pregnant women it is defined as < 11.0 g/dL. The hemoglobin concentration cutoff level that defines anemia varies by age, gender, physiological status, smoking status, and the altitude at which the assessed population lives.
The primary cause of anemia is iron deficiency, a condition caused by inadequate intake or low absorption of iron, the increased demands of repeated pregnancies—particularly if not well spaced (e.g., fewer than 36 months between pregnancies)—and loss of iron through menstruation. Other causes of anemia include vitamin deficiencies (such as a deficiency of folic acid or vitamin A), genetic disorders, malaria, parasitic infections, HIV, tuberculosis, common infections, and other inflammatory conditions. While iron deficiency anemia (IDA) accounts for about onehalf of all anemia cases, it often coexists with these other causes.
Iron deficiency anemia is most common during pregnancy and in infancy, when physiological iron requirements are the highest and the amount of iron absorbed from the diet is not sufficient to meet many individuals’ requirements (Stoltzfus and Dreyfuss 1998). Anemia’s effects include increased risk of premature delivery, increased risk of maternal and child mortality, negative impacts on the cognitive and physical development of children, and reduced physical stamina and productivity of people of all ages (Horton and Ross 2003). Globally, IDA annually contributes to over 100,000 maternal deaths (22 percent of all maternal deaths) and over 600,000 perinatal deaths (Stoltzfus, Mullany, and Black 2004). Key anemia control interventions include promoting a diversified diet, iron-folic acid (IFA) supplementation during pregnancy, iron fortification of staple foods, prevention and treatment of malaria, use of insecticide-treated bed nets, helminth prevention and control, delayed cord clamping, and increased birth spacing.
Maternal Anemia in Ethiopia
The prevalence of anemia among pregnant women in Ethiopia is 22 percent, making it a moderate public health problem as defined by WHO standards1 (CSO and ICF International 2012). Regarding anemia severity, the majority of cases among pregnant and breastfeeding or non-pregnant women reported in the 2011 Ethiopia Demographic and Health Survey (EDHS) are classified as mild or moderate.2 Only 1.2 percent of anemia cases in pregnant women and less than one percent in breastfeeding or non-pregnant women are diagnosed as severe. Hookworm infection and short birth intervals may put pregnant women at increased risk of anemia. In a study carried out at an antenatal clinic in southwest Ethiopia, pregnant women who were anemic were twice as likely to have hookworm infection and three times more likely to have given birth less than 24 months before their current pregnancy (Belachew and Legesse 2006).
Falter Points in Women's Consumption of Iron-Folic Acid During Pregnancy
WHO recommends that all pregnant women receive a standard dose of 30–60 mg iron and 400 μg folic acid beginning as soon as possible during gestation (WHO 2012a). Ideally, women should receive iron-containing supplements no later than the first trimester of pregnancy, which means ideally taking 180 tablets before delivery. It is important to note, that many countries aim for women to receive 90 or more tablets during pregnancy.
Figure 1 on the following page shows a decision-tree analysis of how well the Ethiopian antenatal care (ANC) system distributes IFA, and identifies four potential points at which the system might falter (highlighted in orange). The figure tracks the number and percentage of women who obtained ANC, those who subsequently received and consumed at least one IFA tablet, and those who consumed the ideal minimum number of tablets.3 All data are based on the EDHS questions that were asked of women who were in a permanent union and had been pregnant in the five years prior to being interviewed4 (CSO and ICF International 2012).
Figure 1. Analysis of Falter Points Related to Distribution and Consumption of IFA through Ethiopia’s ANC Program in 2011, Women of Reproductive Age (15–49 years) n=16,515
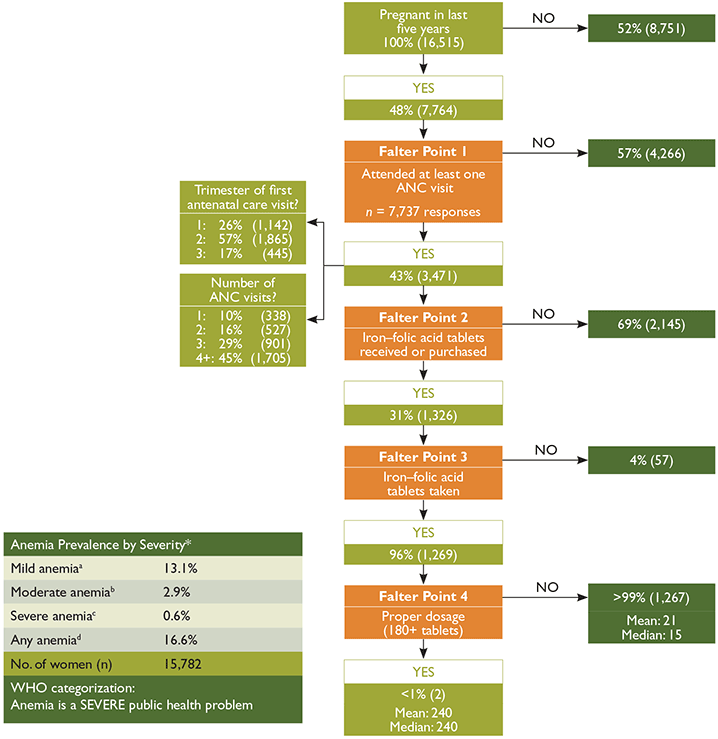
Main Conclusions: Given low coverage rates, ANC is currently an underutilized platform for distributing IFA in Ethiopia. Among women who were pregnant in the last five years, had at least one ANC visit during their last pregnancy, and took at least one IFA tablet, less than one percent received and took the ideal minimum number of tablets. Overall, the most important shortcomings are Falter Points 2 and 4, though Falter Point 1 presents an important obstacle as well. Both supply and demand are likely constraints.
*Percentage of women 15–49 years based on Hemoglobin levels, Hb (g/dL)
aNPW 10.0≤Hb≤11.9, PW 10.0≤Hb≤10.9 b NPW 7.0≤Hb<9.9, PW 7.0≤Hb≤9.9 cNPW Hb<7.0, PW Hb<7.0 dNPW Hb<12.0, PW Hb<11.0
Non-responses, no data (NR/ND) were recoded to “No” for “IFA tablets received?” and “IFA tablets taken?” and to zero for “Number of tablets taken?”. Anemia prevalence data are provided as a reference point, signaling the general order of magnitude of the anemia public health problem. The ANC utilization data is based on self-reported data of women 15–49 years in permanent unions and pertains to their last pregnancy in the last five years prior to the DHS.
Source: Calculations and anemia levels are from the Ethiopia Demographic and Health Survey (2011).
Many supply-side aspects—including both adequacy of IFA tablet supplies and technical knowledge and practices of ANC providers—need to be considered when assessing how well an ANC program delivers IFA. In addition, Falter Point 4 in Figure 1 clearly shows that the provision of IFA tablets to a pregnant woman is a necessary but not sufficient condition for the woman to consume the tablets, particularly at the ideal minimum. Thus, demand-side factors also play a critical role in determining the coverage and effectiveness of this program. These include whether or not women seek antenatal care and the timing and number of visits, as well as the extent to which women are aware of the significance of anemia and IFA, ask for IFA tablets, and comply with the IFA regimen.
Understanding the relative significance of each falter point enables them to be prioritized for more in-depth analysis, providing a first step in an evidence-based approach to systematically improving the program. The DHS does not collect information on the number of IFA tablets received by women. In the case of Falter Point 4, this lack of data creates ambiguities that make it impossible to fully understand whether shortcomings of the system relate primarily to supply- or to demand-side factors. Despite this limitation, the decision-tree analysis presented in Figure 1 still enables prioritizing the falter points for more in-depth analysis and action at the national, district, and health center levels.
Analysis of Falter Points
Falter Point 1:
Did not attend at least one ANC visit
Fifty-seven percent of women did not obtain at least one ANC visit.
ANC’s relatively low coverage underutilizes the platform as a vehicle for providing IFA.
Falter Point 2:
Did not receive or purchase at least one IFA tablet
Of the women with at least one ANC visit, 69 percent did not receive or purchase any IFA.
There are several system/supply-side performance shortcomings that need additional analysis, including: (1) Inadequate supply (e.g., stock outs); (2) Provider knowledge; (3) Provider practices that would lead to failure to provide IFA. As Figure 2 shows, this is the second most important falter point among all women in Ethiopia.
Unfortunately, the EDHS does not report the source(s) of the IFA tablets women received or purchased. It is likely that women who attend ANC are more likely to be aware of, to value and to be inclined to also take IFA, regardless of where they obtain them. Thus we would expect there to be a high correlation between the number of women who had at least one ANC visit and those who received or purchased IFA, which is consistent with the data. While 21 percent of Ethiopian women who received or purchased IFA did not have any ANC visits (not shown), we cannot ascertain whether or not those who received ANC care obtained their IFA from their ANC provider. What is known, however, is that women who have one or more ANC visits and who did not receive any IFA, constitute a missed opportunity to reduce the risk of anemia among a high-risk population.
Falter Point 3:
Did not take at least one IFA tablet
Of the women who received IFA, four percent did not consume any tablets.
This demand-side constraint is relatively small and may be due to women not understanding the significance of anemia and/or the significance of IFA.
This misunderstanding may reflect: (1) inadequate provider counseling and follow-up; (2) women’s beliefs about actual or possible side-effects; or (3) sociocultural factors.
Falter Point 4:
Did not consume 180 or more IFA tablets
Of the women who received and took IFA, more than 99 percent did not consume the ideal minimum of 180 IFA tablets
This is a combination of supply and/or demand-side factors. Figure 1 sheds some light on two possible causes of this falter point: 74 percent of women who received ANC began their care after the first trimester, and the 55 percent who had less than WHO’s recommended four ANC visits during their last pregnancy may have started their ANC too late or may not have had enough visits to receive 180 tablets (given IFA distribution protocols). Both of these are likely contributing factors, but further research is needed to establish their relative importance, as well as the significance of other possible causes.
Globally, research has found that other common causes of Falter Point 4 include: (1) providers do not have access to adequate supply; (2) women do not receive adequate tablets because they have little access to care, or start ANC late, or do not have enough ANC visits making it difficult to obtain 180 tablets (given IFA distribution protocols); (3) providers do not provide adequate counseling or follow-up; (4) women do not adhere to the regimen, which may be due to difficulty in remembering to take the tablets daily, not knowing all the tablets are necessary, fear of having a big baby, side effects, tablet-related issues (taste, size, color, coating, packaging/storage problem). Further research is needed to determine the specific reasons for this falter point in Ethiopia.
Table 1. ANC Coverage and IFA Tablets Taken During Last Pregnancy in the Last Five Years by Region, Residence, and Wealth, Ethiopia, 2011
| Characteristic | No. Women with a Live Birth-Last 5 Years | Received ANC | Took 1 + IFA Tablet | ||
|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | ||
| Region | |||||
| Residence Area | |||||
| Wealth | |||||
| Tigray | 530 | 342 | 64.5% | 178 | 33.6% |
| Affar | 78 | 27 | 34.6% | 18 | 23.3% |
| Amhara | 1,991 | 813 | 40.8% | 378 | 19.0% |
| Oromiya | 3,116 | 1,232 | 39.5% | 368 | 11.8% |
| Somali | 198 | 50 | 25.3% | 39 | 19.9% |
| Benishangul-Gumuz | 92 | 38 | 41.3% | 21 | 23.3% |
| SNNP | 1,634 | 663 | 40.6% | 245 | 15.0% |
| Gambela | 31 | 18 | 58.1% | 9 | 27.5% |
| Harari | 19 | 11 | 57.9% | 6 | 32.9% |
| Addis Ababa | 193 | 182 | 94.3% | 75 | 38.7% |
| Dire Dawa | 26 | 16 | 61.5% | 7 | 27.8% |
| Urban | 1,188 | 914 | 76.9% | 323 | 27.2% |
| Rural | 6,720 | 2,477 | 36.9% | 1,021 | 15.2% |
| Lowest 40% | 3,435 | 1,033 | 30.1% | 443 | 12.9% |
| Highest 40% | 3,154 | 1,741 | 55.2% | 655 | 20.8% |
| National Average | 7,908 | 3,392 | 42.9% | 1,344 | 17.0% |
Analysis by Sociodemographic Variables and Trends Over Time
A comparison of 2005 and 2011 DHS data reveals that Ethiopia has substantially expanded health system coverage of pregnant women: (1) the coverage of ANC increased from 28.5 percent to 42.9 percent; (2) the percent of women with at least one ANC visit who took iron tablets or syrup increased by 63 percent, from 10.4 percent to 17 percent; which may have contributed to (3) the prevalence of anemia among women 15–49 years falling by 38 percent, from 26.6 percent to 16.6 percent. Despite this progress, Ethiopia’s performance on these indicators remains weak by global standards.
Table 1 shows significant variations in the coverage of the Ethiopian ANC Program and consumption of IFA tablets across regions, urban/rural residence, and wealth. For example, while nationwide ANC coverage is 43 percent, the rate varies dramatically by region, from 25 percent in Somali to 94 percent in Addis Ababa. The inequalities between urban and rural areas and in wealth between the lowest 40 percent and the highest 40 percent of Ethiopian households both vary by a factor of nearly two.
Analysis by Geographic Region
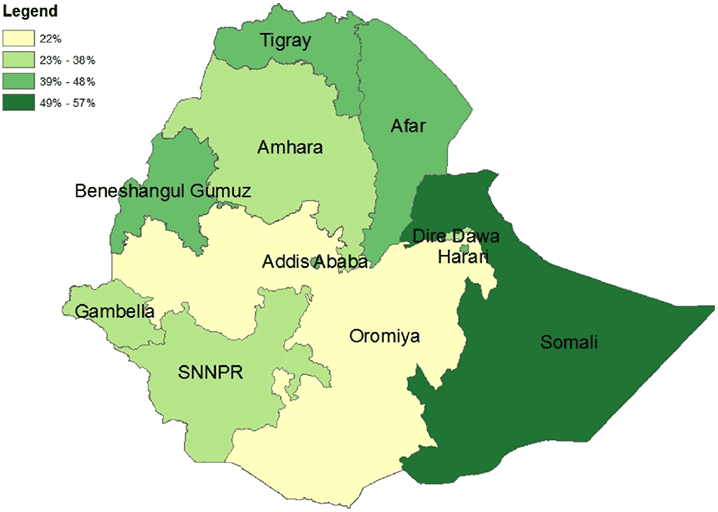
The map in Figure 2 shows how the percentage of women who received at least one ANC visit and reported receiving at least one IFA tablet varies by region. Oromiya has the lowest percentage, but none of the regions exceeds 60 percent. Surprisingly, Somali, which has a large nomadic population, has the highest percentage, 57 percent.
Although Ethiopia’s 16.6 percent nationwide anemia prevalence rate puts it in the “mild” public health problem category, there are regions in which anemia remains a “moderate” or “severe” problem. Moreover, many regions have prevalence rates that are only marginally below the “moderate” threshold. The highest anemia rates in 2011 were observed in Somali, Affar, and Dire Dawa at 44, 34.8, and 28.8 percent, respectively. By this criterion, Somali should be the top regional priority, followed by Affar and Dire Dawa. Additionally, Somali and Dire Dawa were the only regions in which prevalence rates increased between 2005 and 2011. This occurred despite the fact that both had more than doubled the percentage of women (with a live birth in the past five years) who took at least one IFA during their last pregnancy over the six year period.
Formative research is required to gain a better understanding of the specific causes of the shortcomings in the falter points listed above and is essential to developing activities to address them and to increasing the ANC-based distribution of IFA.
Figure 2. Percentage of Women Who Had at Least One ANC Visit and Received at Least One IFA Tablet by Region, Ethiopia, 2011
As would be expected, an inverse relationship emerges between the regional prevalence of anemia and the coverage of ANC (shown in Figure 3); however, this relationship severely constrains the possibility of using ANC as the primary platform for distributing IFA to areas where the need for IFA is greater (i.e., prevalence is higher). From the graph we also see that the relationship between IFA/ANC and anemia is not very strong, underlining the fact that IFA distribution through ANC is only one of many interventions or processes that has an effect on anemia prevalence rates.
Figure 3. Prevalence of Anemia Among Women 15–49 and Coverage of ANC by Region, Ethiopia, 2011
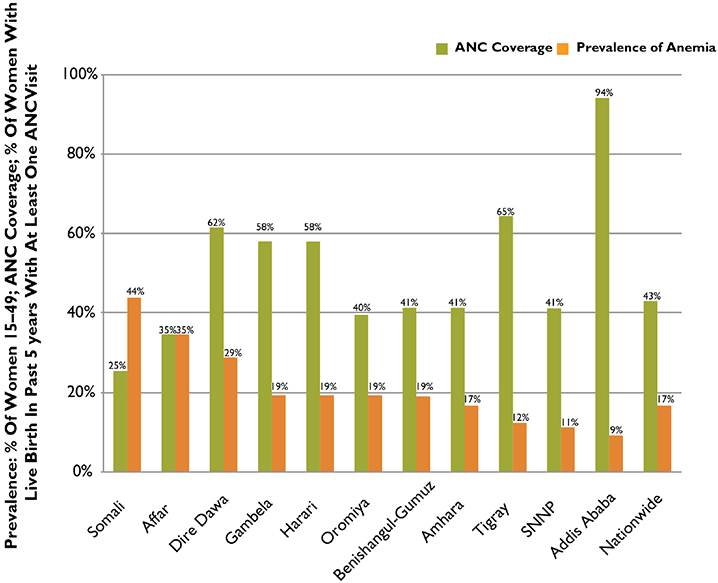
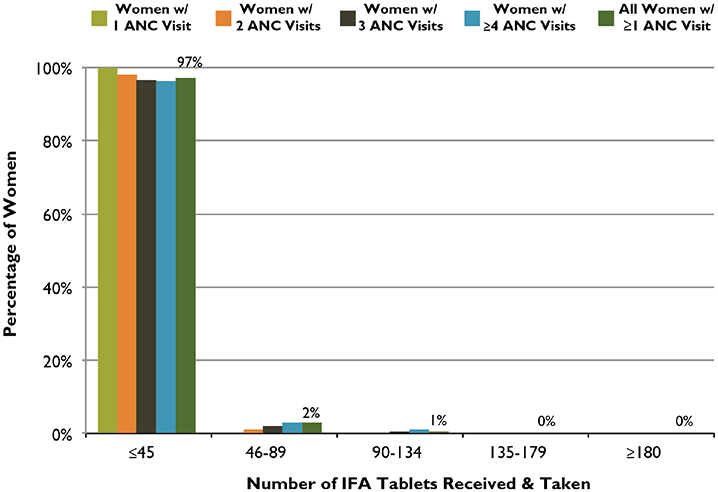
Figure 4. ANC Distribution of IFA Tablets: Number of Tablets Received and Taken According to Number of ANC Visits, Ethiopia, 2011
Ethiopia will need to improve the coverage of ANC—especially in those regions with the greatest prevalence of anemia—if the distribution of IFA through ANC is to be more effective in helping to reduce anemia. Given that improving the coverage of ANC is a mid- to long-term proposition, other strategies for reducing anemia should be considered, including the introduction of communitybased distribution of IFA.
Analysis by Number of ANC Visits
Figure 4 shows the relationship between the number of IFA tablets taken by women who had at least one ANC visit and the number of ANC visits they had during their last pregnancy. Among women who had at least one ANC visit, 97 percent took at most one-quarter (45) of the ideal minimum number of IFA tablets. Women with more visits were generally likely to receive and take more IFA tablets, but only when the number of tablets received and taken was more than 45. Even among those women who received IFA tablets and had four or more visits, 96 percent took at most only one-quarter of the ideal minimum number of IFA tablets.
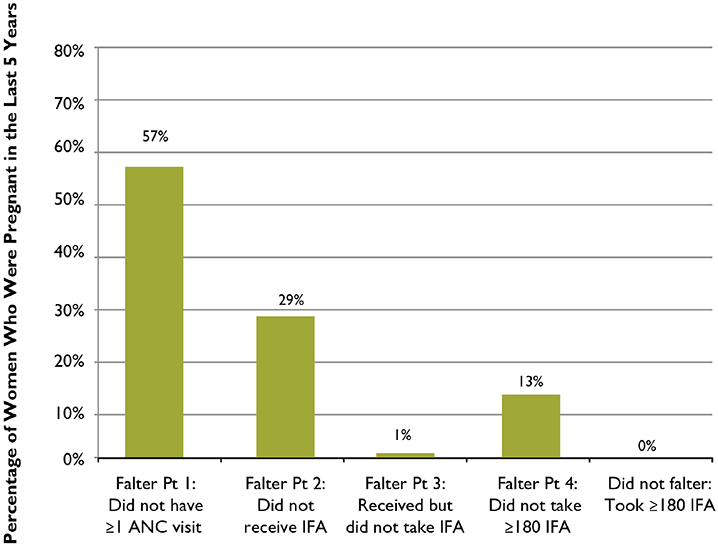
Figure 5. The Relative Importance of Each of the Falter Points in Ethiopia: Why Women Who Were Pregnant in the Last Five Years Failed to Take the Ideal Minimum of 180 IFA Tablets
Overall Conclusions and Recommendations
Figure 5 presents the obstacles amongst all women, including those who did not receive ANC during their pregnancy, to taking the ideal minimum number of IFA tablets. In Ethiopia, Falter Points 1, 2, and 4 appear to be the greatest barriers to women taking the ideal minimum number of IFA tablets. Improving the delivery of IFA supplementation in Ethiopia relies on increasing access to ANC (addressing Falter Point 1) but also on identifying and addressing program gaps in IFA supply management and health workers’ practices (to improve performance at Falter Points 2 and 4). Modifying some women’s long term adherence behaviors, and addressing other points mentioned above, under “Analysis of Falter Points,” may also lead to more women taking a minimum of 180 tablets (addressing Falter Point 4).
This rapid assessment of the distribution of IFA tablets through Ethiopia’s ANC Program suggests that there is substantial room for improvement in the supply- and also demand-sides of the program. Ethiopia has made notable progress in steadily reducing its maternal mortality rate (MMR) from 871 maternal deaths per 100,000 live births in 2001/2002 to 673 in 2006/2007 to its current (2012) level of 350 (MOFED 2010; WHO 2012b). To accelerate its progress toward achieving its fifth Millennium Development Goal (MDG5) to improve maternal health and reach an MMR of 267 or lower, Ethiopia should conduct additional studies to identify and prioritize the causes of the as yet unrealized potential of its ANC-based IFA distribution— especially in Somali, Oromiya, and Affar—so that it can begin addressing these shortcomings. Given that 92 percent of the Ethiopian women who had at least one ANC visit received their care in a public facility (CSO and ICF International 2012), Ministry of Health facilities should be the focus of this work. Improving the distribution of IFA through the ANC program is an important strategy to prevent and control anemia in Ethiopia, and to improve the nutrition and health status, as well as the mental and physical capacity of women of reproductive age.
Footnotes
1 The WHO categorizes the severity of anemia as a public health problem according to its prevalence: <5%, no public health problem; 5–19.9%, mild; 20–39.9% moderate; ≥40%, severe.
2 The DHS hemoglobin levels used to diagnose the severity of anemia in non-pregnant women are different than those specified by the WHO. The DHS cutoffs for pregnant and non-pregnant women in hemoglobin grams/deciliter are: Mild 10.0-10.9 (P) 10.0-11.9 (NP); Moderate 7.0-9.9 (P) 7.0-9.9 (NP); Severe <7.0 (P) <7.0 (NP); Any <11.0 (P) <12.0 (NP).
3 The EDHS asked about IFA tablets or capsules; this brief refers to all types as “tablets.”
4 The EDHS provides a population-based, nationally representative sample of all women in Ethiopia.
References
Belachew, Tefera, and Yared Legesse. 2006. “Risk factors for anemia among pregnant women attending antenatal clinic at Jimmy University Hospital, southwest Ethiopia.” Ethiopian Medical Journal 44(3): 211-220.
Central Statistical Agency (CSO) [Ethiopia] and ORC Macro. 2006. Ethiopia Demographic and Health Survey 2005. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Agency and ICF International.
CSO [Ethiopia] and ICF International. 2012. Ethiopia Demographic and Health Survey 2011. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Agency and ICF International.
Federal Ministry of Health (FMOH) [Ethiopia]. 2004. National guideline for control and prevention of micronutrient deficiencies. Addis Ababa: Family Health Department, Federal Ministry of Health, Government of Ethiopia.
Galloway, Rae. 2003. Anemia Prevention and Control: What Works. Washington, DC: USAID.
Horton, S., and J. Ross. 2003. “The Economics of Iron Deficiency.” Food Policy 28(1):51–75.
Ministry of Finance and Economic Development (MOFED) [Ethiopia]. 2010. Ethiopia: 2010 MDGs Report. Trends and Prospects for Meeting the MDGs by 2015. Addis Ababa, Ethiopia: Ministry of Finance and Economic Development.
Stoltzfus, R. J., and M. L. Dreyfuss. 1998. Guidelines for the Use of Iron Supplements to Prevent and Treat Iron Deficiency Anemia. Washington, DC: ILSI Press.
Stoltzfus, R. J., L. Mullany, and R. E. Black. 2004. “Iron Deficiency Anemia.” In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. M. Ezzati, A. D. Lopez, A. Rodgers, and C. J. L. Murray, eds. Geneva: World Health Organization.
World Health Organization (WHO). 2012a. “Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women.” Geneva: WHO.
WHO. 2012b. Indicator and Measurement Registry version 1.6.0. Maternity mortality rate. (August 28, 2012).