Executive Summary
Background and Methods
Anemia is an urgent public health problem that affects children and women throughout the life course and results in a high burden of morbidity and mortality. Caused by multiple factors, anemia requires a package of mitigating interventions. The main approaches to managing anemia include malaria prevention, diagnosis, and treatment; helminth prevention and control; and nutrition-related interventions. The Ghana Health Service, in collaboration with stakeholders and with the support of the USAID/MOST project, developed the five-year Integrated Anaemia Control Strategy in 2003, which targeted pregnant women, preschool and school-aged children with food-based interventions as well as interventions to control malaria and helminth infection. The Ghana Health Service requested SPRING to provide support in conducting a landscape analysis describing the anemia situation in Ghana. The objectives are to better understand the factors that contribute to anemia, track the progress of anemia-related programs, and identify and reconcile problems and gaps.
This landscape analysis used multiple strategies to capture the current situation in Ghana with respect to anemia and anemia-related policies and programs. The analysis compiled data from two national surveys—the 2008 and 2014 Ghana Demographic and Health Surveys (GDHS). In addition, a systematic review of published and gray literature was conducted to find any relevant smaller-scale surveys or studies that reported additional, but not necessarily nationally representative, data. Relevant databases and websites were searched for documents, reports, and policies already in place. Finally, to gain further understanding into the association between anemiarelated factors and outcomes, logistic regressions were run to calculate odds ratios for both children under five and women, using data from the 2014 GDHS.
Findings and Next Steps
In Ghana, the overall prevalence of anemia is 66 percent among children age 6–59 months (2014) and 42 percent among women of reproductive age (2014). There was a moderate reduction in the prevalence of anemia in children and women between 2008 and 2014; 12 percent for children and 16 percent for women. Among children, the decline tended to disproportionately benefit less vulnerable groups, especially children from the highest quintiles of wealth and children whose mothers’ had primary, middle, or secondary educations. Among women, the decline was seen uniformly across urban and rural areas and geography and wealth subgroups. Anemia significantly declined among pregnant as well as breastfeeding and non-pregnant women.
Malaria is endemic in Ghana, with a prevalence of 26.7 percent among children (2014), and is likely a large contributor to anemia. There is limited data on the prevalence of malaria at the national level among women, although data from regional surveys and intervention studies showed the prevalence of malaria in the range of 7 to 58 percent (2000-2012) among pregnant women and 39 to 49 percent (2001-2007) among non-pregnant women.
Data on other key risk factors for anemia are not measured in the GDHS, but studies have found the prevalence of helminthic infections to range from 8.5 to 45 percent (2006-2013), iron deficiency from 4 to 68 percent (2000- 2013), and vitamin A deficiency from 15 to 76 percent (2002-2013). From our regression analysis, factors associated with an increased risk for anemia among children 6–59 months in 2014 are fever in the past two weeks and stunting, while older children (24–59 months), children whose mothers have more years of education, and those who are in the richest quintiles are at a lower risk of being anemic. Although less data are available for women, the risk of anemia associated with geographic region appears lower among women in the highest quintiles and women who have more years of education. Several key factors for anemia (malaria, micronutrient deficiencies, and helminth infections) were not included in the regression analyses because of lack of data. In summary, the main risk factors for anemia in Ghana are malaria, helminth infections, and micronutrient deficiencies.
In general, there have been notable improvements in anemia-related programming, specifically with iron–folic acid (IFA) supplementation and malaria prevention in pregnancy. There is high coverage of IFA supplementation (93 percent, 2014) in pregnant women. Preventive measures against malaria for both children and pregnant women have improved between 2008 and 2014. Intermittent preventive treatment of malaria in pregnancy (IPTp) increased by 24 percentage points between 2008 and 2014. On the other hand, deworming coverage did not improve from 2008 to 2014, remaining low, at 39 percent among pregnant women, based on 2014 data. Deworming coverage is less than universal for children (38 percent). There is moderate vitamin A coverage in under-five children (65 percent, 2014).
Other efforts to improve micronutrient intake include fortification and infant and young child feeding programs, Mass fortification of vegetable oils with vitamin A is widespread (with over 95 percent of oil adequately fortified), but improvements in the production process for wheat flour fortification with multiple micronutrients are needed, alongside promotion of fortified food use and its coverage. The exclusive breastfeeding rate declined from 63 to 52 percent between 2008 and 2014. Family planning efforts can amplify the impact of supplementation and prevention programs, specifically among adolescent girls aged 15–19 years. Targeting this group can delay age of first pregnancy and increase birth intervals, which would allow women to maintain adequate iron stores. It is observed that the unmet need for family planning is highest among girls 15–19 years (51 percent, 2014). Introducing intermittent IFA supplementation to this vulnerable population could also reduce anemia and improve iron stores prior to conception. The water, sanitation and hygiene situation has generally improved. The overall sanitation situation with respect to improved toilet facilities has improved for Ghana, although there have been implementation challenges like the increase in open defecation in rural areas, from 29 to 33 percent between 1990 and 2012. There is also a corresponding decrease in provision of improved water facilities with associated increases in the use of bottled/sachet water.
The strategy on anemia, developed more than 10 years ago, will need to be updated to reflect the latest understanding of anemia and programming realities. Apart from programs, strengthening the antenatal care (ANC) system creates an opportunity to provide an integrated package of services to pregnant women (IFA, IPTp, and deworming). Specific programs targeting intermittent IFA supplementation for menstruating women and preschool and school-age children or point-of-use fortification for young children are not being implemented on a national scale, although Ghana Health Service is currently evaluating the feasibility of both these interventions. Delayed cord clamping has been included as part of active management of the third stage labor but has not been implemented. There is a need for a multi-sectoral effort to synchronize programs from the different sectors.
With input and additional support from stakeholders, the current integrated strategy for addressing anemia can be updated to reflect the latest understanding of anemia-related interventions and scaled to those areas most affected by anemia.
Background
Anemia is a disorder that occurs when there are insufficient circulating red blood cells or inadequate red cell hemoglobin or tissue oxygenation. Anemia in pregnancy increases maternal and infant mortality; impairs cognitive and physical development of children; and reduces physical stamina and work productivity. Anemia is one of the most common public health problems in the world today. The burden of anemia disproportionately affects young children and women of childbearing age (particularly pregnant women). Globally, 43 percent of children under five, 38 percent of pregnant women, and 29 percent of non-pregnant women 15–49 years of age are anemic (Stevens et al. 2013). Anemia is most widespread in Central and West Africa, where 71 percent of children under five years old, 48 percent of non-pregnant women, and 56 percent of pregnant women are affected (Stevens et al. 2013).
Anemia is a complex condition with many causes. One of the biggest contributors to anemia is low intake of absorbable dietary iron to meet the needs of the body, particularly during adolescence and pregnancy, and during periods of rapid growth in childhood. Iron deficiency anemia (IDA) alone contributes to over 100,000 maternal deaths and 591,000 perinatal deaths each year (Stoltzfus, Mullany, and Black 2004). IDA is more common during pregnancy and in infancy, when iron requirements are higher. Nutrient deficiencies, such as vitamin A, folic acid, vitamin B12, and zinc also directly or indirectly contribute to anemia. Other causes of anemia include malaria, helminthic infections, chronic infections like HIV and tuberculosis, causes related to reproduction and contraception, and genetic disorders such as thalassemia and sickle cell anemia (Balarajan et al. 2011). These underlying causes are functionally linked and act synergistically to exacerbate the effects of anemia. Due to anemia’s multifactorial causation, all anemia prevention and treatment activities should utilize a multi-sectoral and integrated approach to identifying specific causes in given settings and populations.
The main approaches to managing anemia include malaria prevention, diagnosis, and treatment; helminth prevention and control; reducing overall infectious disease burden; and dietary interventions (Balarajan et al. 2011). Additionally, social behavior change communication strategies support these approaches. Indirect approaches related to nutrition-sensitive agriculture, family planning, water, sanitation, and hygiene, and infant and young child feeding are also part of anemia prevention and control efforts.
Malaria control and prevention includes activities related to vector control, distribution and use of long-lasting insecticide-treated nets (ITNs), indoor residual spraying (IRS), and control of mosquito larvae proliferation. It also includes intermittent preventive treatment of infants, children, and pregnant women. Effective diagnosis of malaria and appropriate treatment can also reduce the burden of anemia. Helminthic infection control involves improving the quality of the water supply, sanitation, and hygiene (WASH), and individual and mass treatment with antihelminthic medication. Reducing the overall infectious disease burden may indirectly reduce anemia by preventing chronic “anemia of inflammation,” which causes a reduction of circulating micronutrients in the plasma (Thurnham and Northrup-Clewes 2007). Preventing nutritional anemia requires adequate intake, absorption, and utilization of minerals and vitamins.
Food-based approaches include modifying food processing and preparation techniques to ensure optimal availability and absorption, prompting the consumption of adequate amounts of iron-rich foods, increasing the bioavailability of iron-rich foods by supporting farmers to grow and market iron-rich crops, mass food fortification, and biofortification of foods. These strategies are preferable in the long-term, and interim interventions are often needed to meet the recommended dietary intake requirements of populations that are not reached using food-based approaches. Point-of use fortification with micronutrient powders (MNPs) or iron tablets or syrups for children can increase iron status and effectively reduce anemia. Furthermore, given the high iron requirements during pregnancy, iron–folic acid (IFA) supplementation is recommended for all pregnant women, and more recently, for all women of reproductive age. Delayed cord clamping is a non-dietary intervention to increase the total body iron content of infants at birth.
The Ghana Health Service (GHS), in collaboration with a multi-sectoral multistakeholder committee and with support from USAID/MOST and UNICEF, developed a five-year Integrated Strategy for Anaemia Control in 2003. The strategy targeted pregnant women, preschool, and school-age children with food-based interventions as well as interventions for control of malaria and helminth infection. However, there is no process under way for updating this strategy.
SPRING supports country and global-level efforts to address anemia. As part of this effort, the GHS requested SPRING to conduct an anemia landscape analysis. The objectives of this landscape analysis of anemia in Ghana are to: 1) better understand the factors that contribute to anemia; 2) identify the current anemia-related policies; and 3) track progress of anemia-related programs. The analysis will contribute to the strategy review and strengthen anemia programming. Discussions with national policymakers and district program implementers can help shape and expand Ghana’s anemia strategy, leading to a revised national anemia strategic plan.
Methods
Multiple strategies were used to capture the current anemia and anemia-related programming in Ghana. Data are captured from the most recent national surveys on anemia prevalence and related behaviors: the 2008 and 2014 Ghana Demographic and Health Surveys (GDHS). The 2008 and 2014 GDHS survey data were collected in the late rainy season (September-November and September-December, respectively). Anemia was assessed in both surveys by taking a capillary blood sample and analyzing hemoglobin levels using HemoCue™. National water and sanitation data were extracted from the WHO/UNCIEF Joint Monitoring Programme for Water Supply and Sanitation (1990-2012).
We conducted a literature review of published and gray literature to find any relevant smaller-scale surveys or studies that reported additional, but not necessarily nationally representative, indicators. Using the Ovid platform, we searched the following databases from inception to September 2014: Ovid MEDLINE, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, CAB Abstracts, and the Global Health Archive. The search terms for anemia, risk factors for anemia, Ghana, and specific population groups were used to create the search strategy. The complete search strategy can be found in appendix A. For the landscape analysis, we included studies published after 2000 (with the exception of an article on neural tube defects that was published in 1992, because it featured data not available in later studies). We also searched Google Scholar for all articles published in 2003 or later, using the following combinations of search terms: anemia and Ghana; malaria and Ghana; iron and Ghana; hemoglobinopathies and Ghana; micronutrient and Ghana and anemia; vitamin A and Ghana and anemia; vitamin B12 and Ghana and anemia; folate and Ghana and anemia; zinc and Ghana and anemia. After removal of duplicates, the titles and abstracts of 1,444 articles were screened, 110 of which were accepted for full text screening. Of the 110, 102 were available as PDFs for full text screening. Relevant data on prevalence of anemia, risk factors for anemia, and anemia-related programs from 43 studies were included. We also included relevant studies that we received from subject area experts.
To assess policies and programs currently in place, we consulted multiple sources of information. We searched the World Health Organization (WHO) Global database on the Implementation of Nutrition Action (WHO 2014a) for information pertaining to Ghana’s policies related to anemia. We included information from the 2008 publication titled Landscape Analysis of Readiness to Accelerate the Reduction of Maternal and Child Undernutrition in Ghana (Brantuo et al. 2009). We searched the website of the SUN Movement (SUN 2013), specifically the page devoted to Ghana and its nutrition policies and programs. We explored the Ghana Ministry of Health and GHS websites for official documents as well as for project and program websites. We also followed up with key stakeholders involved in national nutrition actions in Ghana for remaining questions on policies and programs emerging from the desk review.
We evaluated the trend in anemia prevalence by looking at changes in overall prevalence among children under five years and women of reproductive age (WRA) between 2008 and 2014, as well as in population subgroups based on geographic or socioeconomic characteristics. We used the two-proportion z test to determine whether the difference between two proportions is significant. We also wanted to understand the determinants of anemia using multiple regressions of various predictors on anemia in both WRA and children under five years, using the 2014 GDHS data. The variables included in the regression model consisted of a combination of socioeconomic factors and various program coverage indicators. Several key factors for anemia (malaria, micronutrient deficiencies, and helminth infections) were not included in the regression analyses because of lack of data.
The findings of the landscape analysis are presented in three sections: 1) trends in anemia prevalence among children and WRA between 2008 and 2014; 2) prevalence of risk factors for anemia, which include malaria, helminth infection, micronutrient deficiencies, and general inflammation, and the status of related policies and programs;1 and 3) description of the policy environment in Ghana. The “Findings” section is followed by a section discussing considerations for national action.
Population-based Survey Findings
Trends in Anemia Prevalence among Children and Women of Reproductive Age between 2008 and 2014
Table 1 compares the anemia prevalence among children for 2008 and 2014. Based on disaggregated data from 2008 and 2014, the anemia burden is significantly greater among children from poor households, rural households, and children of less educated mothers.
Whereas anemia among children slightly increased between the 2003 and 2008 DHS (from 76 to 78 percent) (GSS, GHS, and ICF Macro 2009), in 2014, the overall prevalence declined modestly, to 66 percent. Notably, however, the decline tended to disproportionately benefit less vulnerable groups. Children from the highest and lowest wealth quintiles experienced a 23 and 9 percent decrease in anemia, respectively, between 2008 and 2014; in 2008, anemia prevalence in the highest quintile was 26 percentage points lower than for those children in the lowest quintile. Additionally, children of mothers who had no education experienced only a 3 percent decrease in anemia, while significant reductions in anemia prevalence were documented among children whose mothers had a primary, middle, or secondary education. Similarly striking was the diversity in decline among regions: in the Northern region anemia rates did not change, whereas in the Ashanti region rates declined by 31 percent.
Table 1 Prevalence of Anemia in Children Age 6–59 Months by Background Characteristics, 2008 (N=2,313) and 2014 (N=2,568)
| Background Characteristic | 2008 (%) | 2014 (%) | Percentage Change, 2008–2014 |
|---|---|---|---|
| Age in months | |||
| Sex | |||
| Residence | |||
| Region | |||
| Mother's education | |||
| Wealth index quintile | |||
| Total | 77.9 | 65.7 | -16 |
| 6-11 | 82.8 | 78.6 | -5 |
| 12–23 | 85 | 76.3 | -10 |
| 24–35 | 79.7 | 66.1 | -17 |
| 36–47 | 75.4 | 61.3 | -19 |
| 48–59 | 70 | 53.2 | -24 |
| Male | 79.1 | 65.5 | -17 |
| Female | 76.6 | 66 | -14 |
| Urban | 67.9 | 57.3 | -16 |
| Rural | 84.1 | 73.6 | -12 |
| Western | 80.4 | 64.7 | -20 |
| Central | 84.5 | 70.2 | -17 |
| Greater Accra | 62.1 | 59.6 | -4 |
| Volta | 78.7 | 69.9 | -11 |
| Eastern | 73.1 | 66.1 | -10 |
| Ashanti | 77.9 | 53.7 | -31 |
| Brong Ahafo | 78.3 | 62.5 | -20 |
| Northern | 81.4 | 82.1 | 1 |
| Upper East | 88.5 | 73.8 | -17 |
| Upper West | 88.2 | 73.8 | -16 |
| None | 82.6 | 79.9 | -3 |
| Primary | 80.5 | 68.7 | -15 |
| Middle | 76.6 | 60.8 | -21 |
| Secondary + | 57.7 | 52.0 | -10 |
| Poorest | 87.4 | 79.5 | -9 |
| Second | 83.6 | 74.7 | -11 |
| Middle | 81.5 | 64 | -21 |
| Fourth | 67.8 | 58 | -14 |
| Richest | 61.2 | 47.1 | -23 |
Sources: GSS, GHS, and ICF Macro 2009; GSS, GHS, and ICF International 2015. Anemia severity is defined as follows: mild, 10.0-10.9 g/dL; moderate, 7.0-9.9 g/dL; severe, <7.0g/dL. N/A=Not available in GDHS 2014 Key Indicators report.
In terms of severity, the greatest declines were in severe (defined as a hemoglobin level below 7.0 g/dL) and moderate (7.0–9.9 g/dL) anemia, whereas mild anemia (10–10.9 g/dL) slightly increased (figure 1).
Figure 1. Anemia Prevalence among Children Age 6–59 Months by Severity, 2008 and 2014
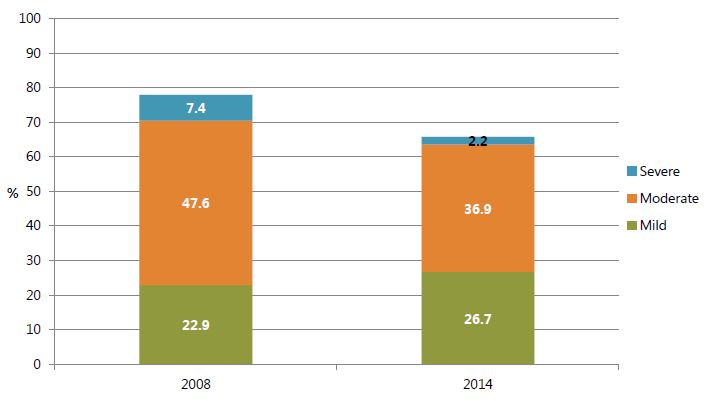
Sources: GSS, GHS, and ICF Macro 2009; GSS, GHS, and ICF International 2015.
The pattern of anemia vulnerability appears to be different among women of reproductive age. The overall prevalence of anemia among women of reproductive age decreased significantly, from 59 to 42 percent between 2008 and 2014. Notably, the decline was seen across urban and rural areas and geography and wealth subgroups. Women in the highest and lowest wealth quintiles experienced similar percentage decreases (30 and 28 percent, respectively). The prevalence rates uniformly declined in all the regions and these decreases ranged from 16 to 47 percent. Between 2008 and 2014, anemia prevalence decreased significantly among pregnant women (36 percent), though a notable 27 percent reduction was also observed among breastfeeding and non-pregnant women.
Table 2. Prevalence of Anemia in Women Age 15–49 by Background Characteristics, 2008 (N=4,757) and 2014 (N=4,644)
| Background Characteristic | 2008 (%) | 2014 (%) | Percentage Change, 2008–2014 |
|---|---|---|---|
| Residence | |||
| Region | |||
| Wealth index quintile | |||
| Education | |||
| Age | |||
| Number of children ever born | |||
| Maternity status | |||
| Total | 58.7 | 42.4 | -28 |
| Urban | 55.3 | 41.2 | -26 |
| Rural | 61.8 | 43.9 | -29 |
| Western | 71.2 | 42.6 | -40 |
| Central | 63.7 | 46.7 | -27 |
| Greater Accra | 50.7 | 42.4 | -16 |
| Volta | 58.1 | 48.7 | -16 |
| Eastern | 58.3 | 38.9 | -33 |
| Ashanti | 59.9 | 40.5 | -32 |
| Brong Ahafo | 57.8 | 36.4 | -37 |
| Northern | 59.3 | 47.5 | -20 |
| Upper East | 48.4 | 39.6 | -18 |
| Upper West | 66.9 | 35.6 | -47 |
| Poorest | 60.7 | 43.7 | -28 |
| Second | 63.0 | 49.3 | -22 |
| Middle | 59.5 | 46.0 | -23 |
| Fourth | 57.9 | 37.3 | -36 |
| Richest | 53.9 | 37.6 | -30 |
| None | 59.9 | 45.5 | -24 |
| Primary | 63.5 | 44.6 | -30 |
| Middle | 58.7 | 40.9 | -30 |
| Secondary + | 51.1 | 40.4 | -21 |
| 15–19 | 63.0 | 47.7 | -24 |
| 20–29 | 57.6 | 43.3 | -25 |
| 30–39 | 58.3 | 38.8 | -33 |
| 40–49 | 56.2 | 41.2 | -27 |
| 0 | 58.7 | 45.0 | -23 |
| 1 | 56.5 | 40.9 | -28 |
| 2-3 | 58.6 | 40.3 | -31 |
| 4-5 | 59.1 | 40.9 | -31 |
| 6+ | 60.6 | 43.9 | -28 |
| Pregnant | 70.0 | 44.6 | -36 |
| Breastfeeding | 61.8 | 45.0 | -27 |
| Neither | 56.6 | 41.3 | -27 |
Sources: GSS, GHS, and ICF Macro 2009; GSS, GHS, and ICF International 2015. Anemia severity is defined as follows: mild, 10.0-11.9 g/dL (10.0-10.9 g/dL for pregnant women); moderate, 7.0-9.9 g/dL; severe, <7.0 g/dL. N/A=Not available in GDHS 2014 Key Indicators report.
In terms of severity, notable declines occurred across all three categories for anemia: severe (defined as hemoglobin level below 7.0 g/dL), moderate (7.0–9.9 g/dL) and mild (10.0-11.9 g/dL or 10.0-10.9 g/dL for pregnant women) anemia, though the decrease in mild anemia was slightly less pronounced (figure 2).
Figure 2. Anemia Prevalence among Women Age 15-49 by Severity, 2008 and 2014
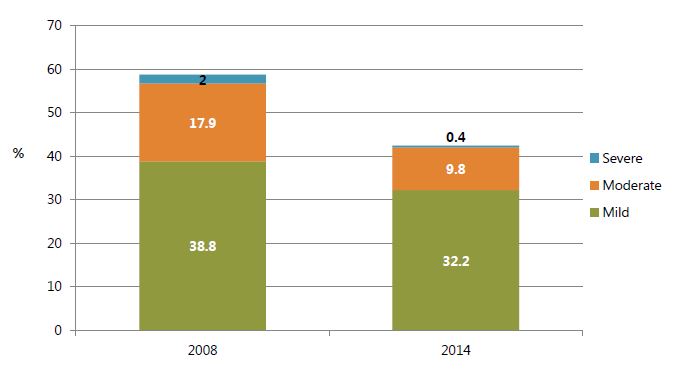
Sources: GSS, GHS, and ICF Macro 2009; GSS, GHS, and ICF International 2015.
Other groups vulnerable to anemia included rural women, pregnant women, and women age 15–19 years. Among DHS surveys for countries conducted in 2005 or after (43 surveys total), a slight majority (56 percent) indicated adolescent females as less anemic than other women of reproductive age.
A comparison between tables 1 and 2, however, indicates notable differences in vulnerability to anemia. Increase in the level of mothers’ education and in the wealth of the household were associated with lower prevalence of anemia in children. In women, however, the relationship between education and wealth did not show the same trend. Women in the lowest quintile of wealth had a lower rate of anemia than women in the second quintile, and those with no education had lower rates of anemia than those with primary school education. Whereas a clear relationship existed between wealth and child anemia, as well as between mother’s education and child anemia, women from the lowest wealth quintile and those with no education fared slightly better than women in the second wealth quintile with a primary education, respectively. Another difference found is that the regions with the highest burden of child anemia are not always the same as those with the highest burden of anemia among women. For instance, in 2014, the Upper West region had the second-highest child anemia prevalence among all regions (73.8 percent) but also the lowest prevalence among women of reproductive age (35.6 percent). The differences between anemia in women and children is not readily explained by the available disaggregated data.
The regression of anemia on various predictors was done to explore whether there were any socioeconomic, demographic, or program coverage characteristics that could explain the high prevalence of anemia in children under five years and WRA (appendix tables B1 and B2). Several key risk factors for anemia (malaria, micronutrient deficiencies, and helminth infections) were not included in the regression analyses because of lack of data. The results are consistent with existing knowledge about anemia causes and vulnerability: among children, some of the most robust predictors of anemia status appear to be age (with older children being less vulnerable than infants) and stunting. It is likely that stunting and anemia coexist due to similar causal mechanisms influenced by socioeconomic and demographic factors. The association between anemia and reporting having a fever in the previous two weeks may be indicative of a general inflammatory process or recently having malaria, as red blood cell destruction from malaria continues to generate anemia for approximately two weeks after initiation of treatment with antimalarials (Menendez, Fleming, and Alonso 2000). Children in the highest wealth quintiles and those with more educated mothers also appear less likely to have anemia. Among WRA, living in wealthier households and having more years of education decreased the odds of having anemia. Similar to the regional results for anemia in table 2, a relationship between subregion and anemia is apparent for women of reproductive age, with those living in Brong Ahafo and Upper East having the lowest odds for anemia. Finally, some variable results should be interpreted with caution due to probable correlation with other key factors not captured in the survey. For instance, the relationships between anemia and being overweight/obese may be the result of associations with other sociodemographic and geographical factors. This cofounding association may also explain the unexpected negative association between anemia and living in an urban residence or having an improved water source. The tables of regression coefficients for children under five and WRA are found in appendix B.
Prevalence of Risk Factors for Anemia and Status of Anemia Control Program
Malaria and Its Prevention and Treatment
Malaria causes anemia both directly by destroying red blood cells and indirectly by decreasing production of new red blood cells (Mohandas and An 2012). In Ghana, malaria is not only a substantial contributor to anemia, but also a significant public health problem in its own right: according to the GHS, malaria is the top cause of child mortality and morbidity. As with anemia, children under five and pregnant women are at highest risk for malaria.
Malaria Prevalence
The most recent national survey of malaria in children was conducted during the 2014 GDHS, which found 26.7 percent prevalence. Figure 3 displays child anemia and malaria prevalence ranked in order of anemia prevalence. Although areas with the highest malaria burdens tend to have a higher prevalence of anemia (Northern, Upper East, and Central), this relationship is inconsistent in some cases. For instance, Upper East has one of the lowest malaria prevalence rates in the country (12 percent), but its anemia burden is the same as that of Upper West, where malaria prevalence is more than three times greater. Infections other than malaria and micronutrient deficiencies are likely to play an even more substantial role in the burden of anemia in regions like Upper East and Greater Accra.
Figure 3. Prevalence of Anemia and Malaria among Children under Five by Region, 2014
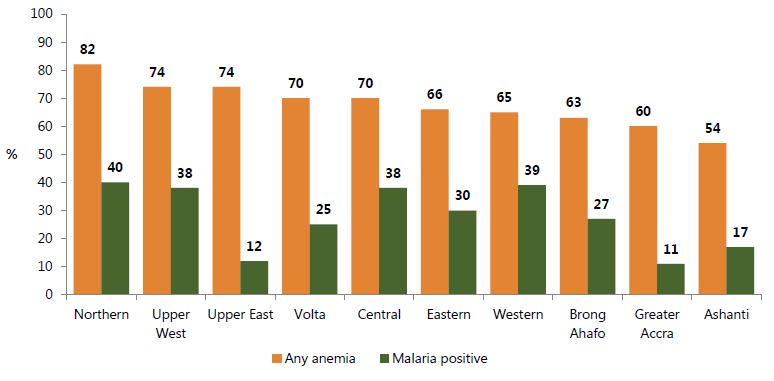
Source: GSS, GHS, and ICF International 2015.
There are no national-level data on the prevalence of malaria and peripheral parasitemia in pregnant women. Our systematic review identified 15 studies that document the prevalence of malaria in pregnant women from different regions in Ghana (appendix table C1). The studies are a mix of cross-sectional surveys (10 studies) and the baseline prevalence from randomized control trials of vitamin A supplementation or antimalarial diagnosis, treatment, and prevention (five studies). The prevalence ranges from 7 percent in Accra to 58 percent in Kassena-Nankana district. The northern districts report a higher prevalence of malaria, as identified by microscopy, as compared to the western or southern areas. There is no discernable trend in malarial prevalence reported in cross-sectional studies as compared to the baseline values in randomized control trials. It would not be unusual to see trials of antimalarial treatment conducted in areas with high malaria prevalence, but the range of prevalence in trials extends from 16.3 to 58 percent, compared to cross-sectional studies, which report a range of 7 to 47.3 percent.
We also found two studies that reported malaria prevalence in women of reproductive age (appendix table C2). The first, published in 2001, was a study of malaria immunity after treatment in adults aged 18–55 years. The study reported a baseline prevalence of 38.8 percent in women age 18–55 years. The second, published in 2007, was a cross-sectional hospital-based study, called the Women’s Health Study, of Accra that assessed the self-reported prevalence of malaria among adult women over 18 years of age. This study found that 48.7 percent of adult women reported having malaria over the past four weeks. There were many more studies that reported the prevalence of malaria in adults, but they did not present disaggregate data on women of reproductive age. There were no studies that evaluated the effect of seasonal change on the prevalence of malaria in pregnant women or women of reproductive age.
Malaria Prevention and Treatment
Some of the strategies for malaria control include use of insecticide-treated nets (ITNs), indoor residual spraying (IRS), intermittent preventive treatment of pregnant women (IPTp), and diagnosis and treatment of malaria with artemisinin-based combination therapy (ACT). The latest figures corresponding to these strategies are presented in figure 4. The prevalence of households owning an ITN was 45 percent in 2008, which increased considerably to 68 percent in 2014. In addition, there was a remarkable improvement in the percentage of children and pregnant women who slept under a bednet the previous night or in a household receiving IRS: an increase from 39 to 54 percent between 2008 and 2014 for children, and from 24 to 50 percent in pregnant women, during the same time frame. The percentage of households owning an ITN or receiving IRS increased from 51 percent in 2008 to 68 percent in 2014. Although more effort is likely needed to attain the target of universal coverage of IPTp, the percentage of women receiving two or more doses of an antimalarial increased from 44 percent in 2008 to 68 percent in 2014.
Increasing treatment-seeking behaviors and resources to adequately test and treat malaria in children, however, appears to be more difficult. In 2014, 78 percent of children with fever sought treatment promptly, and only 34 percent of those children received an antimalarial. Although some fever cases are not caused by malaria, the low level of malaria testing suggests that treatment of fever with ACTs is done mainly in the presence of positive malaria test results.
Figure 4. Selected Malaria Control Indicators, 2014
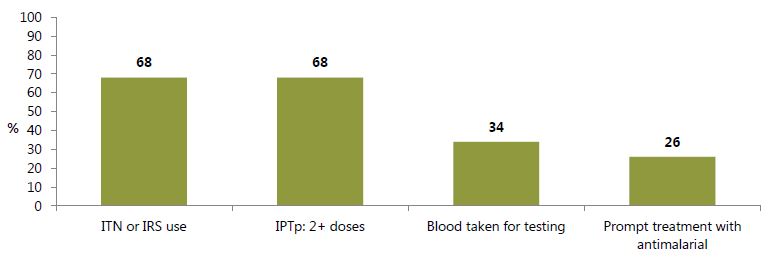
Definitions: ITN or IRS use: Percentage of households owning at least one ITN and/or that received IRS in the past 12 months. IPTp: Among women with a live birth in the past two years, percentage who received sulfadoxine-pyrimethamine (SP)/Fansidar 2 or more times during their last birth. Blood taken for testing: Among children under five with fever in the past two weeks, percentage whose blood was taken for testing. Prompt treatment for ACT: Among children under five with fever in the past two weeks, those who received an ACT the same or next day. Source: GSS, GHS, and ICF International 2015.
As of 2014, the National Malaria Control Programme (NMCP) plans to institute another preventive measure targeted to children in the areas most affected by malaria (Upper West, Northern, and Upper East regions): seasonal malaria chemoprevention. Similar to IPTp, ACTs will be administered intermittently, but to children three to 59 months, and during months where malaria transmission is highest (Ardayfio 2014).
Helminth Infection and Its Control
Hookworm and other worms can lead to anemia by causing gastrointestinal blood loss, poor nutrient absorption, inhibition/suppression of appetite, and general inflammation. Hookworm and other helminthic infections can lead to gastrointestinal blood loss, poor nutrient absorption, inhibition/suppression of appetite, and general inflammation, which can aggravate iron deficiency and anemia, particularly in children (Albonjco et al. 1998) and pregnant women (Steketee 2003).
Prevalence of Helminthic Infections
Although there are no national data on prevalence of helminthic infection, our systematic review identified seven subnational or hospital-based studies that report the prevalence of helminthic infection in the range of 0.7–45 percent in different age groups (appendix table C3). According to the reported studies, hookworm infection was the highest in Kintampo (Brong-Ahafo region) and lowest in the Kassena-Nankana district (Upper East region). Water-based schistosomiasis infection was reported in two studies in the Brong Ahafo and Northern regions, with a prevalence of 6.7 and 12.3 percent, respectively.
Helminth Infection Control
Deworming coverage has not improved in recent years, with 42 percent and 38 percent of children 6–59 months receiving deworming drugs in 2008 and 2014, respectively (Figure 5).
According to the most recent DHS data, coverage of deworming programs for pregnant women has also stagnated, with 35 percent and 39 percent receiving drugs in 2008 and 2014, respectively (Figure 5).
Figure 5. Deworming during Pregnancy and Routine Deworming, 2008 and 2014
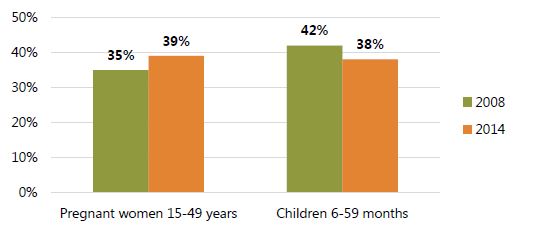
*Given in past 6 months for children and during pregnancy of last birth for women; DHS reports deworming among children 6-59 months. Source: GSS, GHS, and ICF Macro 2009; GSS, GHS, and ICF International 2015.
Micronutrient Deficiency and Its Control with Fortification and Supplementation
Deficiencies in micronutrients can lead to anemia because they are an integral part of red blood cells (iron) (Abbaspour, Hurrell, and Kelishadi 2014), are required for production of new red blood cells (iron, folate, vitamin B12) and transport of iron throughout the body (vitamin A) (Michelazzo et al. 2013), or are required for formation and maturation of red blood cells (zinc, folate, vitamin B12) (Simpson et al. 2010).
Prevalence of Micronutrient Deficiencies
In general, nationally representative micronutrient deficiency data are scant and rarely collected, but in developing countries deficiencies tend to be widespread. Our systematic review found regional and subnational data from cross-sectional studies as well as the pre-intervention baseline micronutrient status reported in intervention trials.
Studies on anemia are far more common than studies on iron deficiency because of the need to measure additional biomarkers like ferritin, soluble transferrin receptor, serum iron, and transferrin saturation. Our systematic review identified 17 studies (from 16 publications) that report the prevalence of iron deficiency (appendix table C4). Iron deficiency in these studies was defined using multiple indicators: ferritin, soluble transferrin receptor, serum iron, and transferrin saturation, and combination of these indicators. When paired with hemoglobin cutoffs to indicate anemia, many authors defined the combined marker as iron-deficiency anemia.
The populations covered included adults, children, pregnant, and non-pregnant women. The data for the prevalence from various regions of Ghana is derived either from baseline values of randomized trials of iron or zinc supplementation (11 studies) or from cross-sectional studies (six studies). The prevalence of iron deficiency (or in some cases, iron-deficiency anemia) reported in these studies ranged from 4 to 68.3 percent. Most of the trials (7 studies from 6 publications) are located in the Brong-Ahafo region, and the baseline prevalence of iron deficiency in the studies from this region ranged from 6.1 to 63 percent (overall 6.1 to 68.3 from all trials). Most of the cohort studies (four studies) have been conducted in the Ashanti region, and the prevalence of iron deficiency in the studies from this region ranges from 5 to 55.6 percent (overall, 4 to 55.6 from all cohort studies). There was no significant association between setting (hospital or community) and reported prevalence of iron deficiency, based on data from different studies in each of the settings.
The search results for vitamin A deficiency is presented in appendix table C5. Our systematic review found 10 studies that assessed the prevalence of vitamin A deficiency, either by serum retinol or by modified relative dose response (MRDR) test. Of these 10 studies, eight were baseline assessments of vitamin A deficiency in randomized controlled trials of vitamin A or micronutrient supplementation, and two cohort studies from the Ashanti and Eastern regions. The prevalence of vitamin A deficiency in two cross-sectional studies of children aged 2–10 years and women aged more than 19 years was 35.6 and 31.7 percent, respectively. From the studies that formed the baseline of randomized controlled trials, the prevalence among children under two was 6.1 and 91 percent in two studies, and ranged from 9.6 to 60.2 among women of reproductive age. Using the MRDR test, the prevalence of vitamin A deficiency ranged from 22 to 91 percent. Using serum retinol as an indicator, the deficiency ranged from 6.1 to 61 percent. In studies on children age 8 months to 10 years, the prevalence of Vitamin A deficiency ranged from 6.1 to 91 percent. In postpartum mothers, the prevalence ranged from 22 to 60.2 percent.
The search results for folate and vitamin B12 deficiency is presented in appendix table C6. One study reported a 30 percent prevalence of vitamin B12 deficiency using low blood levels of B12 as the indicator. Folate deficiency was defined by serum folate, and the prevalence of folate deficiency ranged from 25.5 to 30 percent. Folate inadequacy is also indicated by rates of neural tube defects. There is no cutoff rate value that indicates when neural tube defects are a public health issue. The focus of folate supplementation or fortification is to reduce neural tube defects incidence using a wide range of food supplementation and fortification strategies. A study from 1992 reported the rate of neural tube defects as 1.15/100 live births; a simulation study reported that the national incidence of neural tube defects could be 6.66/100 live births. Both of these studies indicate that folate deficiency is a cause for concern, as it can be presumed that insufficient folate intake can contribute to anemia.
The search results for zinc deficiency are presented in appendix table C7. In our systematic review, five studies defined zinc deficiency either by blood or hair zinc levels. These studies were all from different regions of Ghana. Hair zinc levels are a poor indicator of zinc deficiency as the sequestration of zinc in the hair serves as a poor proxy for body zinc levels, and thus results with this measure are not included. Using serum or plasma zinc as an indicator, the prevalence of zinc deficiency ranged from 19.3 to 85 percent. The wide range of values may be representative of the inherent variation in serum and plasma zinc levels, which makes it difficult to use blood levels of zinc as an indicator of the level of underlying deficiency that needs correction.
Micronutrient Deficiency Prevention and Control
Strategies to increase micronutrient intake fall into two major categories: improving diets through food-based nutrition, and micronutrient supplementation. While promoting dietary diversity is an important strategy to improve nutritional status, sub-Saharan Africa dietary patterns are lacking in absorbable amounts of vitamins and minerals (Ecker, Weinberger, and Qaim 2010). Micronutrient supplementation is required for those population groups who cannot meet their daily requirements for micronutrients through diet alone, which can include populations with particularly high micronutrient requirements (e.g., adolescent girls, pregnant women, young children) and food-insecure populations.
Mass Food Fortification and Biofortification
Large-scale food fortification has also been embraced by the Government of Ghana in response to widespread micronutrient deficiencies found in population surveys done in the first half of the 1990s. The fortification standards for vegetable oil are largely being met: a 2011 nationally representative market survey found that 95 percent of its samples were adequately fortified. Fortification of wheat flour, however, has proven more challenging.
Widespread use of adequately fortified wheat flour requires work on both the supply and demand side. For instance, Ghana’s Food and Drug Authority found that in 2011, only 23 percent of the wheat flour samples they tested were fortified according to the standard, and earlier surveys found wide variability in the level of flour fortification (both very low and very high levels of iron). Efforts to increase awareness of the benefits of fortified flour have been undertaken by the Ministries of Health and Women and Children (the latter through school activation programs), although Nyumuah and colleagues have noted that manufacturers have not invested in similar promotion efforts for their own fortified flour products (Nyumuah et al. 2012). Whether or not the enforcement of standards improves, because of the low consumption of wheat in Ghana, flour fortification alone cannot meet the micronutrient requirements for the entire population. However, enforcement could be a useful adjunct strategy when combined with other programming.
Biofortification of crops (breeding crops to increase nutritional value) is another food-based strategy being tested in other areas of the world. Thus far, biofortified orange-fleshed sweet potatoes have demonstrated increasing vitamin A levels in trials in Bangladesh, Mozambique, and Uganda (Turner et al. 2013; Low et al. 2007; Hotz et al. 2012). Millet and beans have also been biofortified with iron in India, Benin, and Rwanda (Kodkany et al. 2013; Cercamondi et al. 2013; Petry et al. 2014). In Ghana, the promotion of orange-fleshed sweet potato has been led by a stakeholder committee chaired by the Council for Scientific and Industrial Research’s Crops Research Institute.
Micronutrient Supplementation
Iron Supplementation
At no time during a woman’s life are iron requirements higher than when she is pregnant, which is why IFA supplementation is recommended by WHO as early as possible during gestation (WHO 2012). SPRING has developed an algorithm that used DHS data to identify four sequential points at which IFA supplementation programs commonly falter. The results of the analysis for Ghana, using data from GDHS, are shown in appendix D. Based on the analysis, the biggest falter point appears to be the consumption of enough tablets—75 percent of women who attended ANC and consumed at least one tablet did not consume IFA for 180 days or more—and from this analysis it is unclear whether supply or failure to adhere is the bigger problem. A renewed focus on adequate IFA consumption in pregnancy is recommended, along with further investigation into its influencing factors. Iron and folic acid are given as separate pills of 60 mg iron and 5 mg folic acid. Since the WHO recommends a daily dose of 400 μg folic acid in pregnancy, and given concerns about decreased efficacy of sulfadoxine-pyrimethamine (SP)/Fansidar with a 5 mg folic acid dose, there is opportunity to replace the separately distributed 60 mg iron and 5 mg folic acid pills with a combined IFA tablet containing 60 mg iron and 400 μg dose of folic acid.
WHO recommends intermittent IFA supplementation for women of reproductive age, who may not receive enough iron through food-based strategies to improve iron stores prior to pregnancy. In areas where childbearing starts early and/or where anemia is a bigger problem among younger women (prevalence greater than 20 percent), adolescents may be targeted for intermittent supplementation (WHO 2011a). Ghana has recently reviewed the WHO guidelines and has recommended their adoption as policy. Plans are underway for consensus building and establishing modalities for implementation. This is captured in the 2015 Programme of Work of the Nutrition Department, Ghana Health Service.
Vitamin A
In 2014, about two-thirds (65 percent) of children aged 6–59 months had received vitamin A in the previous six months, which is higher than the coverage in 2008 (56 percent).
Micronutrient Powders
One method of improving the micronutrient content of young children’s diets are point-of-use fortification with micronutrient powders (MNPs), which contain a powder mix of micronutrients that are packaged in single-dose sachets and can be added directly to any semi-solid complementary food prepared in the household without substantially affecting the taste or color of the food. WHO recommends the use of MNPs in settings where anemia prevalence is greater than 20 percent to reduce iron deficiency and improve anemia among infants and children 6–23 months of age. In malaria-endemic areas, MNPs should be delivered in conjunction with measures for malaria prevention, diagnosis, and treatment (WHO 2011b). Evidence from efficacy studies conducted in different parts of the world suggest that MNPs are as efficacious as iron drops in reducing and preventing anemia when added to complementary foods, reducing anemia by 31 percent and reducing iron deficiency by 51 percent among young children in settings with both high and low anemia prevalence (De-Regil et al. 2013, CDC-UNICEF and HF-TAG 2013). Studies in Ghana have shown the acceptability and efficacy of MNPs (Adu-Afarwuah et al. 2008, Zlotkin et al. 2001). The Sprinkles Global Health Initiative has conducted randomized controlled trials and bioavailability studies in collaboration with the Kintampo Health Research Centre in Ghana to show the feasibility and effectiveness of MNPs as an anemia-reduction strategy. In a study of 313 Ghanaian children age 6–12 months, the prevalence of anemia in the intervention groups after a year of treatment was 10 percent compared to 31 percent in the control group (Adu-Afarwuah et al. 2008). Although there are some concerns that iron supplementation may increase the risk for malaria, in a randomized controlled trial specifically designed to test the safety of MNPs in the Brong Ahafo region there was no difference in malaria incidence between children receiving MNPs with iron and those receiving MNPs without iron (Zlotkin et al. 2013). Because the trial included malaria prevention and control measures, these findings are consistent with the WHO recommendation that “in malaria-endemic areas, the provision of iron should be implemented in conjunction with measures to prevent, diagnose and treat malaria,” (WHO 2011b).
General Inflammation and Its Control
Malaria and helminth are infections that result in anemia directly. Malaria causes anemia by the destruction of red blood cells; helminthic infections cause anemia by causing blood loss from the intestines (Nemeth and Ganz 2014). Although not a direct cause of anemia, water, sanitation, and hygiene (WASH) also impact the health status of Ghanaians. A number of diseases and infections, such as diarrhea and lower respiratory infections, affect child growth and development through the mechanism of chronic inflammation and environmental enteropathy (Ngure et al. 2014). General inflammation indirectly impacts iron metabolism; one of the better-known pathways for this is by the general increase in the hormone hepcidin. Increase in hepcidin results in lower iron absorption and decreased release of iron from iron stores in the reticuloendothelial system. Basic hygiene reduces the risk of infection, thereby reducing nutritional losses incurred by infection and also reducing inflammation that suppresses hemoglobin formation (Humphrey 2009). Handwashing in older infants reduces infection risk and improves nutritional status as effectively as it does for their mothers. Provision of improved sanitary facilities also reduces transmission of helminthic infections (Ngure et al. 2014).
Water, Sanitation, and Hygiene Interventions for General Inflammation
Recent trend estimates from the WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation, indicate, however, that the problem of open defecation persists. Although open defecation marginally decreased between 1990 and 2012, the drop was largely driven by population shifts from rural to urban areas. In fact, open defecation in rural areas actually increased, from 29 percent in 1990 to 33 percent in 2012, as seen in figure 6. Thus, although the sanitation situation has improved for Ghana overall, renewed focus on the most disadvantaged areas for sanitation is recommended (WHO/UNICEF 2014).
Figure 6. Coverage Estimates of Sanitation Facilities in Ghana, 1990 and 2012
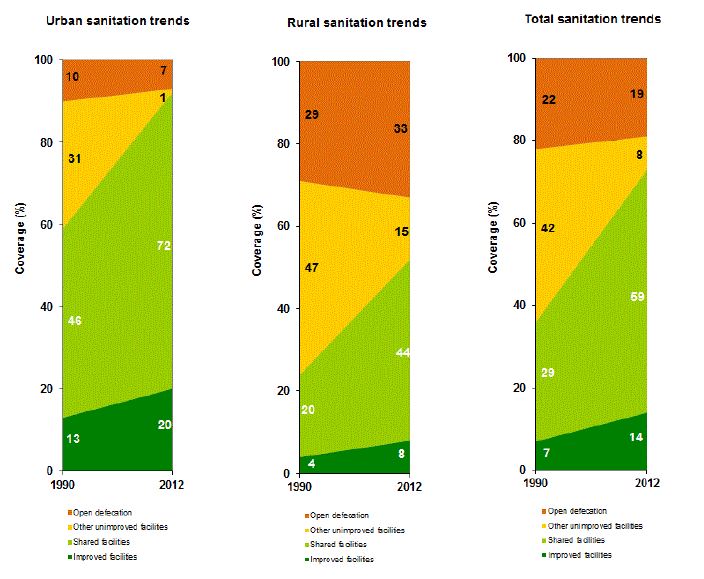
Source: WHO/UNICEF 2014
The 2011 Multiple Indicator Cluster Survey (MICS) found that the majority (76 percent) of households lacked even a place where handwashing could occur; only 12 percent had a place for handwashing, soap, and water when surveyed (GSS 2011). According to the 2014 DHS, 60.1 percent of the households in Ghana have access to an improved source of drinking water, including a piped source within the dwelling, yard, or plot; a public tap, standpipe, tube well, or borehole; a hand pump, protected well, or protected spring; and rainwater. This has decreased from 77.3 percent in 2008. Among the sources, 29.8 percent of the households in 2014 use bottled or sachet water as compared to 13.6 percent in 2008. The households that have an improved toilet facility that is not shared with other households increased from 11.3 percent in 2008 to 13.6 percent in 2014. In 2014, among households in which the place for handwashing was observed, 39.2 percent of households had soap and water for handwashing, and 37 percent of households had no water, soap, or other cleansing agent for handwashing.
Other Programs That Affect Anemia Status
These programs include food-based nutrition strategies, maternal, infant and young child feeding practices, and family planning programs.
Food-Based Nutrition Strategies
The most recent nationwide analysis of dietary quality and quantity (for both children and adults) was conducted in 2009 by the World Food Programme (WFP). The study found that 5 percent of the population was food-insecure, with an additional 9 percent vulnerable to food insecurity. The remaining proportion was generally characterized as consuming starches, meat, and vegetables daily. Food insecurity was significantly higher in the rural north (Northern, Upper West, and Upper East) (Biederlack and Rivers 2009), and in 2012, WFP reported that about 26 percent of these three regions were food-insecure (Hjelm and Dasori 2012). The School Feeding Program, which is headed by the Ministry of Local Government and Rural Development, provides daily meals. The most recent available Annual Operating Plan reported that in 2012, meals contained less than 30 percent of the recommended intake of iron on average, but meals supplied by the WFP (which works in the more food-insecure northern regions) contained 90 percent (Ministry of Local Government and Rural Development, Ghana, 2013).
Infant and Young Child Feeding Practices
WHO recommends exclusive breastfeeding for the first six months of life followed by continued breastfeeding until 24 months in combination with safe and nutritionally adequate complementary foods (WHO and UNICEF 2003). The infant and young child feeding (IYCF) practices recommended by WHO will have a positive impact on anemia prevalence among children by reducing micronutrient loses, preventing gastrointestinal infection, and ensuring adequate dietary intake. In addition, prolonged lactational amenorrhea may increase birth spacing and reduce the mother’s risk of becoming anemic (see “Family Planning” section) (Kramer and Kakuma 2004).
Selected breastfeeding indicators for 2008 and 2014 are presented in figure 7. The country’s exclusive breastfeeding rates have declined, from 63 percent in 2008 to 52 percent in 2014. Continued breastfeeding after one year is nearly universal in Ghana, while rates for early initiation and continued breastfeeding after two years increased slightly in 2014.
Figure 7. Selected WHO/UNICEF Indicators on Breastfeeding Practices in Ghana, 2008 and 2014
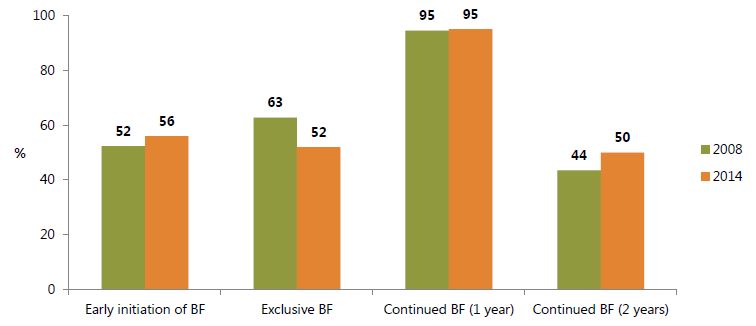
Notes: BF: Breastfeeding. Early initiation of BF: Among children born in the previous 2 years, percentage breastfed within one hour of birth. Exclusive BF: Among children 0–5 months old, percentage currently fed nothing other than breast milk. Continued BF (1 year): Among children 12–15 months old, percentage currently breastfed. Continued BF (2 years): Among children 20–23 months old, percentage currently breastfed. Source: GSS, GHS, and ICF Macro 2009.
Complementary feeding is needed after six months of exclusive breastfeeding, when breast milk alone cannot meet the nutritional requirements of an infant. WHO recommends that after six months, all infants and toddlers receive iron-rich complementary foods, including meat products and/or iron-fortified foods. This supplements breast milk, which should be continued until the child is two years of age (WHO and UNICEF 2003). On the whole, appropriate complementary feeding practices in 2014 have room for improvement (figure 8). Moreover, it appears that these IYCF practices are not entirely driven by food availability: although the northern regions (Northern, Upper East, and Upper West) have higher proportions of poor and food-insecure households (Hjelm and Dasori 2012), they were not always the worst in terms of children receiving the minimum meal frequency or dietary diversity. This suggests a need to promote behavior change among both food-secure and food-insecure areas.
Figure 8. Selected Complementary Feeding Indicators among Children 6–23 Months, 2014
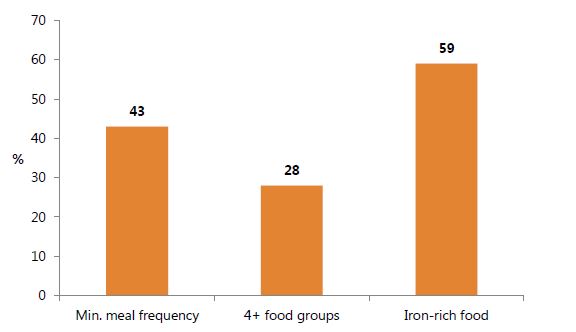
Notes: All questions refer to consumption the day and night previous to the survey. Minimum meal frequency is defined according to WHO/UNICEF guidelines; see http://data.unicef.org/nutrition/iycf for more information. Iron-rich foods include meat, organ meats, fish/seafood, and/or eggs. Source: GSS, GHS, and ICF International 2015.
Family Planning
The timing of pregnancy can be an especially important influence on anemia prevalence among women of reproductive age, as additional iron requirements for pregnancy can be exacerbated in adolescence, where iron requirements are higher than for older women. In addition, childbirth itself can lead to blood loss and if pregnancies are timed too closely together, the capacity for the body to replenish red blood cells is diminished. Because of these factors, the shifting of fertility preferences and use of family planning may influence anemia trends (Begum and Dewey 2010).
In Ghana, the contraceptive prevalence rate barely improved between 2008 and 2014 (figure 9). Still, as figure 10 indicates, the proportion of women who wanted but were not currently using a contraceptive method to space or limit births decreased, most markedly in the 15–19 age group (62 percent in 2008 to 51 percent in 2014). This may be partly due to increased access to family planning, in addition to changing fertility preferences. However, as figure 10 also indicates, adolescents need continued special attention, as the perceived need for family planning remains the highest among this cohort in contrast with other age groups.
Figure 9. Percentage of Married Women Currently Using Contraception (Contraceptive Prevalence Rate), by Education Level and Total, 2008 and 2014
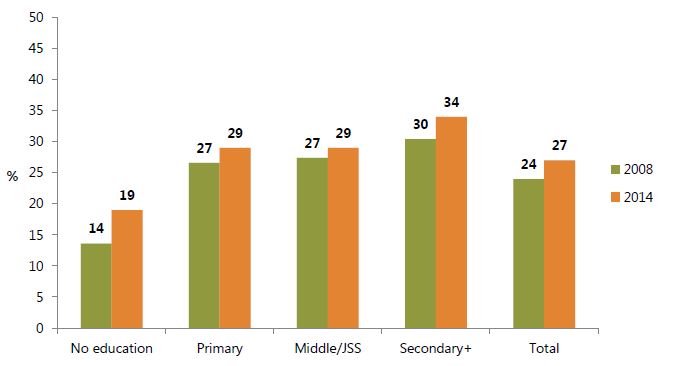
Sources: GSS, GHS, and ICF Macro 2009; GSS, GHS, and ICF International 2015.
Figure 10. Percentage of Married Women with Unmet Need for Family Planning, by Age and Total, 2008 and 2014
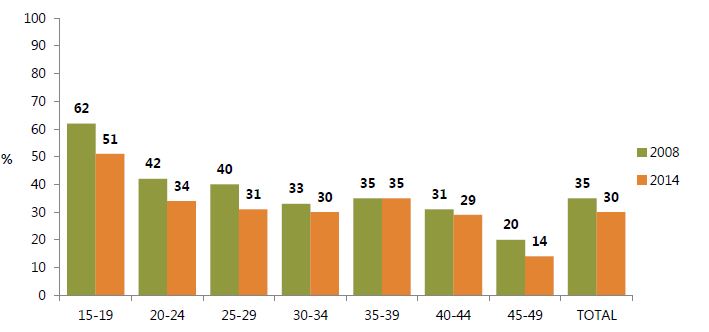
Sources: GSS, GHS, and ICF Macro 2009; GSS, GHS, and ICF International 2015.
Delayed Cord Clamping
Delayed cord clamping is an important intervention that allows the passage of blood from the placenta to the baby and reduces the risk of iron deficiency in infancy (McDonald et al. 1996). WHO recommends late cord clamping (approximately one to three minutes after birth) unless the neonate is asphyxiated and needs to be moved immediately for resuscitation (WHO 2014b). Ghana has adopted the policy of delayed cord clamping, which should be done while initiating simultaneous essential newborn care. GHS has included the practice in its job aids for active management of the third stage of labor (Ghana Health Service and JSI Focus Region Health Project, n.d.). At present there are no data available on the practice of delayed cord clamping in Ghana.
Policy Environment in Ghana
Policies and strategies from different sectors influence the risk factors for anemia, and hence, the prevalence of anemia in the population. In this section, we will discuss some of the policies related to anemia followed by a discussion of the historical efforts and opportunities for multi-sectoral integration.
National Policies for Anemia
Given the multifactorial causation of anemia, there are multiple policies from various sectors that may influence the programs for the prevention and control of anemia. These policies cover a wide range of age and population groups. The Guidelines Adaptation Task Team of the GHS has recently reviewed the WHO guidelines pertaining to micronutrients such as supplementation of IFA and vitamin A, and home fortification with MNPs. The task team has recommended continuation or adoption of some guidelines, namely vitamin A supplementation for infants and children 6–59 months of age, daily iron and folic acid supplementation in pregnant women, intermittent iron and folic acid supplementation in menstruating women and in preschool and school-age children, home fortification of foods with multiple micronutrient powders among infants and children 6–23 months, and wheat and maize flour fortification with sodium iron ethylenediaminetetraacetic acid. For home fortification, the task team will further deliberate whether this intervention should be rolled out nationwide or in a phased approach, with due consideration paid to issues of logistics, supply chains, behavior change, and monitoring and evaluation. There is a need to encourage a stakeholder meeting to foster a policy and program environment for adoption in an integrated fashion with multi-sectoral input. The challenge before the Government of Ghana includes consensus building and establishing modalities for implementation across sectors. This effort is captured in the 2015 Programme of Work of the Nutrition Department, Ghana Health Service.
Ghana’s National Nutrition Policy (NNP) has three objectives: 1) to increase coverage of high-impact nutrition-specific interventions that ensure optimal nutrition of Ghanaians throughout their life cycle, with special reference to maternal health and child survival; 2) to ensure high coverage of nutrition-sensitive interventions to address the underlying causes of malnutrition; and 3) to reposition nutrition as a priority multi-sectoral development issue in Ghana (Government of Ghana 2013). The policy outlines evidence-based interventions for different life stages, including for women in childbearing age, newborns, infants and pre-schoolers, school-age children and adolescents, and mass interventions for the general population, like salt iodization. The policy also addresses interventions from sectors that indirectly contribute to anemia such as WASH, agriculture and food security, social protection and safety nets, and education.
Ghana’s National Malaria Control Programme (NMCP) of 2008–2015 enumerates a number of targets: universal coverage with ITNs (defined as one net for every two persons); insecticide residual spraying of one-third of households; universal coverage of pregnant women with intermittent preventive treatment in pregnancy (IPTp); universal rapid diagnostic/microscopy testing for malaria; and prompt and effective treatment with artemisinin-based combination therapies (ACTs). The NMCP also conducted free mass distribution campaigns for ITNs from 2010 to 2012 and has increased focus on ANC and immunization clinics and schools. Major external development partners that assist the NMCP in addressing malaria include the Global Fund, the UK Department for International Development (DFID), and the President’s Malaria Initiative (President’s Malaria Initiative 2013).
Ghana’s Safe Motherhood Service Protocol also recommends daily IFA (60 mg of iron and 0.5 mg of folate) as part of its Focused Antenatal Care Package (Ghana Health Service 2008). The guidelines for malaria in pregnancy under the NMCP recommend at least three doses of SP for IPTp (Government of Ghana 2014). There is evidence that the 5 mg dose of folic acid (5 mg/day) reduces the efficacy of the chemoprophylaxis with SP (Peters et al. 2007). Thus, guidelines for malaria in pregnancy in Ghana recommend a folic acid dose of 400 μg, and advise against giving 5 mg of folic acid concurrently with SP administration.
Ghana’s current Child Health Policy does not specify presumptive and routine deworming as key interventions to be administered during childhood, but it does mention that deworming drugs can be administered through the immunization system (Ministry of Health, Ghana, 2008). Additionally, the GHS has collaborated with the Ghana Educational Service to administer deworming drugs to schoolchildren (Bosumtwi-Sam 2007). The National Reproductive Health Policy and Standards and the National Safe Motherhood Protocol recommend administering deworming drugs to pregnant women during the second trimester and later (Ghana Health Service 2003; Ghana Health Service 2008). Routine deworming for all pregnant women is not policy. Pregnant women are administered deworming medicines only when there is indication of worm infestation, and this is done after the second trimester.
The Child Health Policy endorses breastfeeding behaviors as well as timely and high-quality complementary feeding in line with these recommendations (except for breastfeeding for HIV-positive mothers, which WHO changed after the policy was enacted). Moreover, Ghana recognizes the International Code of Marketing of Breast Milk Substitutes, and official breastfeeding policies were enacted into law in 2000 (Ministry of Health, Ghana, 2008). The Ghana Health Service has an IYCF strategy based on the WHO recommendations. The GHS has developed and incorporated age-appropriate feeding guidelines into the Child Health Record Booklet, upon which basis caregivers are counseled during growth promotion. The UNICEF Community-IYCF and Essential Nutrition Actions training packages have both been adapted for Ghana and are being used to update the knowledge of service providers on maternal, infant, and young child nutrition. Child Health Policy states that all children 6–59 months shall receive a high dose of vitamin A biannually through the immunization system, Child Health Promotion weeks, and school health programs in addition to health facility contacts (Ministry of Health, Ghana, 2008).
Adolescents represent an age group at higher risk for anemia. The Government of Ghana’s Adolescent Reproductive Health Policy of 2000 has a broad objective of increasing the access of young people to reproductive health information and services. One of the targets in the policy is to reduce teen pregnancies (in people younger than 20 years) by 80 percent.
According to Nyumuah and colleagues’ (2012) account of the industrial food fortification movement through 2011, the Ghana Standards Board designates fortification standards for vegetable oil (fortified with vitamin A) and wheat flour (fortified with vitamins A, B1, B1, niacin, B12, folate, zinc, and iron). Promotion of fortified food and other foods rich in micronutrients is done by GHS staff, who are trained by the National Food Fortification Programme. Provision of daily meals is a part of the School Feeding Program, which is headed by the Ministry of Local Government and Rural Development. It serves selected deprived schools in all regions of Ghana and targets primary school children (ages 5–13). A guiding principle of the program is to locally source the meals and hire private caterers to provide them, and guidance has been provided on ensuring adequate nutrient daily allowances.
Programs to address both food availability and knowledge of nutritious foods are thus required to meet the recommended nutrient intake requirements sufficient to prevent micronutrient deficiencies and anemia. Promotion of production and consumption of micronutrient-rich crops and foods by agricultural extension services, is outlined by the Ministry of Food and Agriculture’s latest Plan (2011–2015). The Women in Agricultural Development Directorate, in particular, has assisted with capacity development for agriculture extension officers on these topics. It should be noted, however, that the amount budgeted for these types of services constituted less than one-tenth of 1 percent of the proposed cost of the entire plan (Ministry of Food and Agriculture, Ghana, 2010).
Although not recognizing anemia specifically, the National Environmental Sanitation Strategy and Action Plan (NESSAP) of 2010–2015 acknowledges water and sanitation as deeply intertwined with the health status of Ghanaians, and lists malaria, diarrhea, and intestinal worms as influenced by poor sanitation. Formulated under the Environmental Health and Sanitation Directorate under the Ministry of Local Government and Rural Development, this plan is similar to the national nutrition plans espoused by the SUN Movement in that they name multiple sectors responsible for promoting WASH (such as the Ministry of Education and Ministry of Health); in addition, it specifies the costs required to meet its vision. There is also considerable overlap between the objectives named in the NESSAP and the latest draft of Ghana’s National Nutrition Policy: both propose increasing handwashing and other sanitary behaviors, promotion of Community-Led Total Sanitation (CLTS), and increasing access to safe water. Much of the NESSAP was based on the individual strategy and action plans developed at the district level, and this process is set to occur every four years (Ministry of Local Government and Rural Development, Ghana, 2010). The Ministry of Local Government and Rural Development appears to have adopted CLTS with the assistance of external development partners (including UNICEF and the Canadian International Development Agency) (GhanaWeb 2014).
The policies pertaining to anemia prevention and control from multiple sectors are only as effective as their implementation.
Integration and Multi-sectoral Approaches for Anemia
Ghana’s NNP (2014-2017) promotes integration of nutrition interventions within existing facility- and community-based maternal, newborn, and child health services. Ghana has a history of implementing integrated anemia control programs. In 1995, World Vision introduced a five-country, 10-year comprehensive micronutrient and health (MICAH) program in Ethiopia, Ghana, Malawi, Senegal, and Tanzania (Berti et al. 2010). In Ghana, the MICAH program was implemented from 1996 to 2005 in the Kwahu South district. The program objectives were: 1) to increase intake and bioavailability of micronutrients (iron, iodine, and vitamin A); 2) to reduce prevalence of diseases that affect micronutrient status (diarrheal, parasitic, and vaccine-preventable); and 3) to build local capacity for delivery systems to improve micronutrient status.
Interventions were integrated into health and nutrition infrastructure. Between 1997 and 2004, an evaluation of the MICAH program found that anemia in women decreased from 43 to 18 percent; from 75 to 31 percent in children under five; and from 63 to 25 percent in pregnant women in the project area (Berti et al. 2010). Malaria in children under five was reduced from 18 to 8 percent and in pregnant women from 6 to 3 percent. Exclusive six-month breastfeeding rates increased from 17 to 49 percent; rates of helminthic infections (hookworm, from 4 to 1 percent and schistosomiasis from 19 to 4 percent) in children 6–12 years were reduced; and access to protected water sources increased from 54 to 79 percent. While all reductions in burden of disease cannot be attributed to the program, MICAH showed the proof of concept for an integrated program of anemia control and prevention.
From 2002 to 2005, the USAID-MOST program provided technical assistance to GHS’s Nutrition Division to initiate a comprehensive approach to anemia in Ghana (MOST 2005). The integrated strategy included three main activities: the drafting of a national anemia strategy, the development of a strategic communication plan; and the coordination of the implementation of both the strategy and the communications plan. An anemia control coordinating committee (ACCC) comprising the GHS and development partners was formed to facilitate the activities. The strategy strengthened and integrated the following interventions in pregnant women: providing IFA supplements; promoting intermittent preventive treatment of malaria and deworming; increasing the use of insecticide-treated nets; and increasing consumption of iron and vitamin C-rich foods. Communication materials and trainings were designed to increase political and public awareness through media campaigns and build capacity of the health staff. For example, Miss Ghana 2004 was recruited to promote messages about anemia prevention in pregnant women to the general public. The integrated program increased awareness and the capacity of the health facilities to deliver anemia program interventions at both the community and health facility levels (MOST 2005).
The recent global and national momentum for multi-sectoral and integrated approaches in nutrition provides an opportunity to address the multiple causes of anemia. One notable development that may impact anemia-related actions is the development of the Ghana National Nutrition Policy, which has been validated by relevant government sectors. The National Development Planning Commission has established a multisector platform for addressing nutrition issues as proposed by the SUN Movement. This platform is known as the Cross-Sectoral Planning Group (CSPG). The strategic decisions of the CSPG are operationalized by the national Nutrition Partners Coordination (NaNuPAC), which is currently coordinated and led by the GHS and has representation from relevant stakeholders, including health, education, food, and agriculture, and development partners and academia.
In Ghana’s current community and governance structures, there are a number of platforms to deliver multiple anemia interventions. In some cases, they work best when sectors operate collaboratively. Examples include:
- Community-based workers, including community health workers, agriculture extension agents, and other community mobilizers. Ground-level workers must provide counseling or services that are complementary, or at least not duplicative. Community health workers can reach populations beyond the clinical setting—whether for MNP distribution, micronutrient supplementation, or pregnancy counseling. As part of its One Million Community Health Worker Campaign (GH1mCHW), Ghana’s goal is to cover 100 percent of its rural population over a 10-year period (Ministry of Health, Ghana, 2014).
- The Focused Antenatal Care Package, which consists of several programs relevant to anemia (Ministry of Health, Ghana, 2008). Because such a high proportion of Ghanaian pregnancies are reached through the ANC system, this platform provides a promising means to refocus efforts on interventions that have had lower coverage (such as deworming drug administration).
- Schools as sites for provision of nutritious meals, deworming tablets, health and nutrition education, and intermittent provision of micronutrient supplementation (such as IFA for adolescent girls). The health and education sectors have an established relationship for promoting health among schoolchildren.
- The agriculture sector has a profound influence on nutritional outcomes for rural populations in particular. Promotion of certain crops or livestock can either improve or worsen anemia among the populations that produce them through the interplay of income generation, women’s empowerment, and availability of micronutrient-rich food for consumption.
While the policies and programs are designed at the national level, coordinating activities and support for multi-sectoral anemia programming and capacity building at the district level are also needed. The prevalence and causes of anemia vary from region to region, as does the status of anemia control programs. Coordination at the district level should involve multiple governmental partners from the different sectors—health, nutrition, agriculture, education, public works, social protection, and women’s and child development—working in conjunction with nongovernmental agencies and the community. With the use of evidence-based tools, district-level officials can prioritize interventions that can address the cause of anemia in their area.
Discussion
Summary of Findings
Although Ghana has shown a reduction in anemia prevalence in children over the last few years, the prevalence of child anemia remains high. There was a significant decline in overall anemia prevalence in children age 6–59 months between 2008 and 2014 (from 78 to 66 percent). Even with the overall reduction in child anemia, some groups and strata—for example, the children from the highest wealth quintile, among those whose mothers have more than a primary school education, and among those from the Ashanti region—have accrued the benefits of anemia control efforts. The prevalence of child anemia remains above 70 percent in the Northern, Central Upper East, and Upper West region, with the prevalence between 2008 and 2014 remaining relatively static (ranging from an increase of 1 percent to a decrease of 17 percent in those regions).The decrease in anemia prevalence in children between 2008 and 2014 benefited those with severe and moderate anemia, while rates of mild anemia increased slightly, which is to be expected as the overall change in prevalence of anemia was not large. The prevalence of anemia in women of reproductive age decreased significantly between 2008 and 2014, especially for those with severe and moderate anemia. Notably, the decline was seen across urban and rural areas and geography and wealth subgroups. Women in the highest and lowest wealth quintiles experienced similar percentage decreases, and there was a uniform decline among the women from the different regions of Ghana.
A national survey that includes measures of the prevalence of a range of infections, nonspecific inflammation, and micronutrient deficiencies would allow for a better understanding of the drivers of anemia in Ghana. There are no recent national survey data on micronutrient deficiencies or helminth infections. Malaria is a known significant contributor to anemia prevalence. There is no consistent relationship between high malaria and high anemia prevalence at the regional level in Ghana. In places where the malaria prevalence is low but anemia prevalence is still significant, other factors, such as iron deficiency and helminthic infections, are likely significant contributors to anemia. Improvements in malaria prevention and treatment likely contributed to the reductions in anemia in children, although given the limited data available, specific conclusions cannot be reached. There has been significant progress for certain malaria control strategies in recent years, such as IPTp coverage among pregnant women, antimalarial treatment of children with fever, and ITN ownership and use by children and pregnant women. These have led to a decrease in the burden of malarial disease in children and pregnant women. The malaria program activities are interlinked with the program of IFA supplementation in pregnancy. The use of a 5 mg dose of folic acid in pregnancy, which is much higher than the WHO-recommended dose of 400 μg, raises concerns of decreased antimalarial efficacy of SP. This suggests an opportunity to replace the separately distributed 60 mg iron and 5 mg folic acid pills with a combined IFA tablet (containing 60 mg iron and 400 μg dose of folic acid) for use in IPTp. This will benefit both the malarial and IFA supplementation programs.
Deworming coverage falls short of the universal goal in children (38 percent) and pregnant women (39 percent), based on 2014 data. However, basic immunization coverage has improved (and possibly reduced anemia-related infections), and the child welfare and care platform may provide a mechanism for increasing coverage of deworming drugs if they are co-administered with vaccines. A renewed effort for deworming is recommended.
At the individual level, IFA and vitamin A supplementation programs are the mainstays of combating anemia. Despite high coverage of IFA supplementation in pregnant women, there is room for improvement. The biggest falter point appears to be the consumption of enough tablets—75 percent of women who attended ANC and consumed at least one tablet did not consume IFA for 180 days or more—and from our analysis, it is unclear whether supply or failure to adhere is the bigger problem. A renewed focus on adequate IFA consumption in pregnancy is recommended, along with further investigation into its influencing factors. Strengthening ANC services, specifically related to access to health facilities and in-service training of health care workers attending to expectant mothers, is needed to ensure that pregnant women receive the package of preventive anemia services. Intermittent IFA supplementation for women of reproductive age, already endorsed by GHS, should be incorporated into updates on the Anemia Control Strategy. Women of reproductive age are at a higher risk of anemia compared to men, and the strategy will ensure women have adequate hemoglobin levels prior to becoming pregnant. The increased coverage of high-dose vitamin A capsules (65 percent) and the fortification of oil with vitamin A may also have played a role in the decline in anemia among children, but it is unlikely that these programs alone would result in the marked reductions of anemia.
Mass fortification programs have a major role to play. Fortification of wheat flour with iron and other nutrients may have reduced anemia prevalence in the small portion of the population reached by this intervention. Mass fortification is a cost-effective way to deliver micronutrients, but it is unlikely that all Ghanaians will have access to fortified foods. Coverage of fortified vegetable oils is almost universal, but improvements for wheat fortification are needed. Furthermore, the low level of wheat consumption in the Ghanaian diet indicates that fortification needs to include more food vehicles and it has to be supplemented with additional micronutrient programs that target the most vulnerable groups.
Infant and young child feeding programs can amplify the impact of supplementation, fortification, and infection control programs. Breastfeeding promotion is an important intervention in the Ghanaian context because of decreasing rates of exclusive breastfeeding. Ghana is taking innovative steps to increase exclusive breastfeeding rates, such as the introduction of a maternity leave policy to promote breastfeeding, in addition to traditional information, education, and communication channels. Ghana has had progressively low rates of early marriage and childbirth and unmet need for family planning in recent history. However, there is still a high percentage of teen pregnancies and a high percentage of adolescent girls reporting an unmet need for family planning. The Government of Ghana’s Adolescent Reproductive Health Policy of 2000 has a broad objective of increasing the access of young people to reproductive health information and services. One of the targets in the policy is to reduce teen pregnancies (in people younger than 20 years) by 80 percent.
Another factor that may have contributed to the reductions of anemia seen in children could be the improvements in water supply and sanitation, which alleviate the infectious burden of disease and inflammation. Further improvements in this area may result in continued reductions in anemia among women and children. The water supply, sanitation, and hygiene sector must improve availability, access, and use of safe water, as well as sanitation and hygiene practices. In particular, gaps in sanitation facility coverage in the rural areas must be filled.
Next Steps
Even with impressive decline in the prevalence of anemia in Ghana, it continues to be a public health problem. Most vulnerable are children under five, adolescent girls 15–19, and pregnant women. Ghana has a number of national policies and regulations in place, but additional policies and strategies, where lacking, should be adopted. Ghana has a history of integrated anemia programming with the MICAH and MOST-sponsored programs. The most recent initiative to comprehensively address anemia in a unified manner was in 2003, when the Nutrition Unit of the GHS prepared a five-year strategic plan that brought together strategies in malaria, fortification, dietary diversification, IFA supplementation in pregnancy, and helminth control. Although some of these activities did occur, the strategy was never fully implemented in an integrated fashion. A concerted effort to review the national strategy will help overcome the political, operational, and technical barriers to implementing an integrated strategy to sustain improvements in anemia reduction. This landscape analysis provides inputs for the review. The gains achieved through implementation of different sectoral programs may be lost if the focus of efforts is diverted to a single program instead of an integrated approach to reduce anemia. It is also important that Ghana engage a broad range of stakeholders and build its capacity to effect such a change. Strong political commitment and continued investment in anemia-related programing is essential for sustaining the gains in anemia reduction achieved in the last decade and improving the nutrition and health status as well as the mental and physical capacity of Ghanaian women and children.
To view the annexes, please download the full report above.
Footnotes
1 Programs that are related to malaria prevention and control, deworming, food-based nutrition, micronutrient supplementation, delayed cord clamping, family planning, and water supply, sanitation, and hygiene
References
Abbaspour, Nazanin, Richard Hurrell, and Roya Kelishadi. 2014. “Review on Iron and Its Importance for Human Health.” Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences 19 (2): 164–74.
Adu-Afarwuah, Seth, Anna Lartey, Kenneth H. Brown, Stanley Zlotkin, André Briend, and Kathryn G. Dewey. 2008. “Home Fortification of Complementary Foods with Micronutrient Supplements Is Well Accepted and Has Positive Effects on Infant Iron Status in Ghana.” The American Journal of Clinical Nutrition 87 (4): 929–38.
Albonjco, M., R. J. Stoltzfus, L. Savioli, J. M. Tielsch, H. M. Chwaya, E. Ercole, and G. Cancrini. 1998. “Epidemiological Evidence for a Differential Effect of Hookworm Species, Ancylostoma Duodenale or Necator Americanus, on Iron Status of Children.” International Journal of Epidemiology 27 (3): 530–37. doi:10.1093/ije/27.3.530
Ardayfio, Rosemary. 2014. “Preventing Malaria during High Transmission Period: Ghana Adopts Seasonal Malaria Chemoprevention.” Graphic Online, May 5. http://graphic.com.gh/news/health/22341-preventing-malaria-during-high-transmission-period-ghana-adopts-seasonal-malaria-chemoprevention.html.
Balarajan, Yarlini, Usha Ramakrishnan, Emre Özaltin, Anuraj H Shankar, and Sv Subramanian. 2011. “Anaemia in Low-Income and Middle-Income Countries.” The Lancet 378 (9809): 2123–35. doi:10.1016/S0140-6736(10)62304-5.
Begum, Khadija, and Kathryn G. Dewey. 2010. “Impact of Early Initiation of Exclusive Breastfeeding on Newborn Deaths.” Technical Brief. Alive and Thrive. http://aliveandthrive.org/sites/default/files/Insight%20Issue%201%20Impact%20of%20early%20initiation_English_test2.pdf.
Biederlack, Lisa, and Jonathan Rivers. 2009. “Comprehensive Food Security & Vulnerability Analysis: Ghana.” Rome, Italy: World Food Programme.
Brantuo, Mary N. A., Wilhelmina Okwabi, Seth Adu-Afuawua, Ernestina Agypong, Nana Tamea Attafua, Gladys Brew, Veronica Gomez, Alice Dawson, and Joseph Ashong. 2009. “Landscape Analysis of Readiness to Accelerate the Reduction of Maternal and Child Undernutrition in Ghana.” In Landscape Analysis on Countries’ Readiness to Accelerate Action in Nutrition, 31–37. SCN News 37. United Nations Standing Committee on Nutrition.
Cercamondi, Colin I., Ines M. Egli, Evariste Mitchikpe, Felicien Tossou, Christophe Zeder, Joseph D. Hounhouigan, and Richard F. Hurrell. 2013. “Total Iron Absorption by Young Women from Iron-Biofortified Pearl Millet Composite Meals Is Double That from Regular Millet Meals but Less than That from Post-Harvest Iron-Fortified Millet Meals.” The Journal of Nutrition 143 (9): 1376–82. doi:10.3945/jn.113.176826.
De-Regil, Luz Maria, Parminder S. Suchdev, Gunn E. Vist, Silke Walleser, and Juan Pablo Peña-Rosas. 2013. “Home Fortification of Foods with Multiple Micronutrient Powders for Health and Nutrition in Children under Two Years of Age (Review).” Evidence-Based Child Health: A Cochrane Review Journal 8 (1): 112–201.
Ecker, Olivier, Katinka Weinberger, and Matin Qaim. 2010. “Patterns and Determinants of Dietary Micronutrient Deficiencies in Rural Areas of East Africa.” Journal of Cooperatives 04 (2). http://ideas.repec.org/a/ags/jlcoop/93867.html.
Ghana Health Service. 2003. “National Reproductive Health Service Policy and Standards: Second Edition.” Accra, Ghana: Ghana Health Service.
———. 2008. “National Safe Motherhood Service Protocol.” Accra, Ghana: Ghana Health Service.
Ghana Health Service and JSI Focus Region Health Project. n.d. “A Guide for Maternal and Newborn Care: Part One: Maternal Care.” Accra, Ghana: Ghana Health Service.
Ghana Statistical Service (GSS). 2011. “Ghana Multiple Indicator Cluster Survey with an Enhanced Malaria Module and Biomarker, 2011: Final Report.” Accra, Ghana: GSS.
Ghana Statistical Service (GSS), Ghana Health Service (GHS), and ICF Macro. 2009. “Ghana Demographic and Health Survey 2008.” Accra, Ghana: GSS, GHS, and ICF Macro.
GhanaWeb. 2014. “Open Defecation Level Drops in Ghana | Regional News 2014-05-22.” Accessed September 10. http://www.ghanaweb.com/GhanaHomePage/NewsArchive/artikel.php?ID=310087.
Government of Ghana. 2013. “National Nutrition Policy. 2014–2017.” Accra, Ghana: Government of Ghana.
Government of Ghana, Ministry of Health. 2014. Guidelines for Case Management of Malaria in Ghana. 3rd ed. Accra, Ghana: Government of Ghana.
Government of Ghana, Ministry of Health. 2014. “National Community Health Worker (CHW) Program.” Accra, Ghana: Government of Ghana. http://www.ghanahealthservice.org/downloads/GUIDELINE%20FOR%20CASE%20MANAGEMENT%20.pdf.
Hjelm, Lisa, and Wuni Dasori. 2012. “Ghana Comprehensive Food Security and Vulnerability Analysis, 2012.” Rome, Italy: World Food Programme.
Hotz, Christine, Cornelia Loechl, Abdelrahman Lubowa, James K. Tumwine, Grace Ndeezi, Agnes Nandutu Masawi, Rhona Baingana, et al. 2012. “Introduction of β-Carotene-Rich Orange Sweet Potato in Rural Uganda Resulted in Increased Vitamin A Intakes among Children and Women and Improved Vitamin A Status among Children.” The Journal of Nutrition 142 (10): 1871–80. doi:10.3945/jn.111.151829.
Humphrey, Jean H. 2009. “Child Undernutrition, Tropical Enteropathy, Toilets, and Handwashing.” The Lancet 374 (9694): 1032–35. doi:10.1016/S0140-6736(09)60950-8.
Humphries, Debbie, Emily Mosites, Joseph Otchere, Welbeck Amoani Twum, Lauren Woo, Hinckley Jones-Sanpei, Lisa M. Harrison, et al. 2011. “Epidemiology of Hookworm Infection in Kintampo North Municipality, Ghana: Patterns of Malaria Coinfection, Anemia, and Albendazole Treatment Failure.” The American Journal of Tropical Medicine and Hygiene 84 (5): 792–800. doi:10.4269/ajtmh.2011.11-0003.
Kodkany, Bhalchandra S., Roopa M. Bellad, Niranjana S. Mahantshetti, Jamie E. Westcott, Nancy F. Krebs, Jennifer F. Kemp, and K. Michael Hambidge. 2013. “Biofortification of Pearl Millet with Iron and Zinc in a Randomized Controlled Trial Increases Absorption of These Minerals above Physiologic Requirements in Young Children.” The Journal of Nutrition 143 (9): 1489–93. doi:10.3945/jn.113.176677.
Kotey, Amos. 2013. “The Causes of Anaemia in Agogo, Ashanti Region, Ghana.” http://ir.knust.edu.gh/handle/123456789/5437.
Kramer, Michael S., and Ritsuko Kakuma. 2004. “The Optimal Duration of Exclusive Breastfeeding: A Systematic Review.” Advances in Experimental Medicine and Biology 554: 63–77.
Low, Jan W., Mary Arimond, Nadia Osman, Benedito Cunguara, Filipe Zano, and David Tschirley. 2007. “A Food-Based Approach Introducing Orange-Fleshed Sweet Potatoes Increased Vitamin A Intake and Serum Retinol Concentrations in Young Children in Rural Mozambique.” The Journal of Nutrition 137 (5): 1320–27.
McDonald, Susan J., Philippa Middleton, Therese Dowswell, and Peter S Morris. 1996. “Effect of Timing of Umbilical Cord Clamping of Term Infants on Maternal and Neonatal Outcomes.” In Cochrane Database of Systematic Reviews. John Wiley & Sons. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004074.pub3/abstract.
Michelazzo, Fernanda B., Julicristie M. Oliveira, Juliana Stefanello, Liania A. Luzia, and Patricia H. C. Rondó. 2013. “The Influence of Vitamin A Supplementation on Iron Status.” Nutrients 5 (11): 4399–4413. doi:10.3390/nu5114399.
Ministry of Food and Agriculture, Ghana. 2010. “Medium Term Agriculture Sector Investment Plan (METASIP).” Ministry of Food and Agriculture, Ghana.
Ministry of Health, Ghana. 2008. “Under Five’s Child Health Policy: 2007-2015.” Accra, Ghana: Government of Ghana.
Ministry of Local Government and Rural Development, Ghana. 2010. “National Environmental Sanitation Strategy and Action Plan (NESSAP).” Accra, Ghana: Government of Ghana.
———. 2013. “Ghana School Feeding Programme: Annual Operating Plan.” Ghana School Feeding Program. Accra, Ghana: Government of Ghana. http://www.schoolfeeding.gov.gh/index.php?option=com_docman&Itemid=112&limitstart=10.
Mohandas, Narla, and Xiuli An. 2012. “Malaria and Human Red Blood Cells.” Medical Microbiology and Immunology 201 (4): 593–98. doi:10.1007/s00430-012-0272-z.
Nemeth, Elizabeta, and Tomas Ganz. 2014. “Anemia of Inflammation.” Hematology/Oncology Clinics of North America 28 (4): 671–81, vi. doi:10.1016/j.hoc.2014.04.005.
Ngure, Francis M., Brianna M. Reid, Jean H. Humphrey, Mduduzi N. Mbuya, Gretel Pelto, and Rebecca J. Stoltzfus. 2014. “Water, Sanitation, and Hygiene (WASH), Environmental Enteropathy, Nutrition, and Early Child Development: Making the Links.” Annals of the New York Academy of Sciences 1308 (1): 118–28. doi:10.1111/nyas.12330.
Nyumuah, Richard Odum, Thuy-Co Caroline Hoang, Esi Foriwa Amoaful, Rosanna Agble, Marc Meyer, James P. Wirth, Lorenzo Locatelli-Rossi, and Dora Panagides. 2012. “Implementing Large-Scale Food Fortification in Ghana: Lessons Learned.” Food & Nutrition Bulletin 33 (4): 293S–300S.
Peters, Philip J., Michael C. Thigpen, Monica E. Parise, and Robert D. Newman. 2007. “Safety and Toxicity of Sulfadoxine/pyrimethamine: Implications for Malaria Prevention in Pregnancy Using Intermittent Preventive Treatment.” Drug Safety 30 (6): 481–501.
Petry, Nicolai, Ines Egli, Jean B. Gahutu, Pierrot L. Tugirimana, Erick Boy, and Richard Hurrell. 2014. “Phytic Acid Concentration Influences Iron Bioavailability from Biofortified Beans in Rwandese Women with Low Iron Status.” The Journal of Nutrition 144 (11): 1681–87. doi:10.3945/jn.114.192989.
Scaling Up Nutrition (SUN). 2013. “Ghana—Scaling Up Nutrition.” Accra, Ghana: SUN. http://scalingupnutrition.org/sun-countries/ghana.
Simpson, Joe Leigh, Lynn B. Bailey, Klaus Pietrzik, Barry Shane, and Wolfgang Holzgreve. 2010. “Micronutrients and Women of Reproductive Potential: Required Dietary Intake and Consequences of Dietary Deficiency or Excess. Part I—Folate, Vitamin B12, Vitamin B6.” The Journal of Maternal-Fetal & Neonatal Medicine: The Official Journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians 23 (12): 1323–43. doi:10.3109/14767051003678234.
Steketee, Richard W. 2003. “Pregnancy, Nutrition and Parasitic Diseases.” The Journal of Nutrition 133 (5): 1661S–1667S.
Stevens, Gretchen A., Mariel M. Finucane, Luz Maria De-Regil, Christopher J. Paciorek, Seth R. Flaxman, Francesco Branca, Juan Pablo Peña-Rosas, Zulfiqar A. Bhutta, and Majid Ezzati. 2013. “Global, Regional, and National Trends in Haemoglobin Concentration and Prevalence of Total and Severe Anaemia in Children and Pregnant and Non-Pregnant Women for 1995–2011: A Systematic Analysis of Population-Representative Data.” The Lancet Global Health 1 (1): e16–25. doi:10.1016/S2214-109X(13)70001-9.
Stoltzfus, Rebecca J., Luke Mullany, and Robert E. Black. 2004. “Iron Deficiency Anaemia.” Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors 1: 163–209.
Thurnham, David, and Christine Northrup-Clewes. 2007. “Infection and Etiology of Anemia.” In Badham, Jane, Michael B. Zimmerman, and Klaus Kraemer (eds). Nutritional Anemia: The Guidebook. Basel, Switzerland: Sight and Life Press.
Turner, Tami, Betty J. Burri, Kazi M. Jamil, and Maleka Jamil. 2013. “The Effects of Daily Consumption of β-Cryptoxanthin-Rich Tangerines and β-Carotene-Rich Sweet Potatoes on Vitamin A and Carotenoid Concentrations in Plasma and Breast Milk of Bangladeshi Women with Low Vitamin A Status in a Randomized Controlled Trial.” The American Journal of Clinical Nutrition 98 (5): 1200–1208. doi:10.3945/ajcn.113.058180.
UNICEF-CDC, and HF-TAG. 2013. “Micronutrient Powders (MNP)—UNICEF-CDC Global Assessment of Home Fortification Interventions.” Geneva, Switzerland: WHO. http://hftag.gainhealth.org/resources/micronutrient-powders-mnp-%E2%80%94-unicef-cdc-global-assessment-home-fortification-interventions-.
World Health Organization (WHO). 2011a. “Guideline: Intermittent Iron and Folic Acid Supplementation in Menstruating Women.” Geneva, Switzlerland: WHO.
———. 2011b. Guideline: Use of Multiple Micronutrient Powders for Home Fortification of Foods Consumed by Infants and Children 6–23 Months of Age. WHO Guidelines Approved by the Guidelines Review Committee. Geneva, Switzerland: WHO. http://www.ncbi.nlm.nih.gov/books/NBK180125/.
———. 2012. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women. WHO Guidelines Approved by the Guidelines Review Committee. Geneva, Switzerland: WHO. http://www.ncbi.nlm.nih.gov/books/NBK132263/.
———. 2014a. “Policies in Ghana | Global Database on the Implementation of Nutrition Action (GINA).” Global Database on the Implementation of Nutrition Action (GINA): Policies in Ghana. Geneva, Switzerland: WHO. https://extranet.who.int/nutrition/gina/en/policies/1440.
———. 2014b. “WHO | Optimal Timing of Cord Clamping for the Prevention of Iron Deficiency Anaemia in Infants.” Geneva, Switzerland: WHO. http://www.who.int/elena/titles/cord_clamping/en/.
World Health Organization (WHO) and UNICEF. 2003. “Global Strategy for Infant and Young Child Feeding.” Geneva, Switzerland: WHO. http://www.who.int/nutrition/publications/infantfeeding/9241562218/en/.
World Health Organization (WHO) and UNICEF Joint Monitoring Programme for Water Supply and Sanitation. 2014. “Estimates on the Use of Water Sources and Sanitation Facilities (1980-2012): Ghana.” Geneva, Switzerland: WHO/UNICEF. http://www.wssinfo.org/fileadmin/user_upload/resources/Ghana.xls.
Yatich, Nelly J., Jiang Yi, Tsiri Agbenyega, Archer Turpin, Julian C. Rayner, Jonathan K. Stiles, William O. Ellis, et al. 2009. “Malaria and Intestinal Helminth Co-Infection Among Pregnant Women in Ghana: Prevalence and Risk Factors.” The American Journal of Tropical Medicine and Hygiene 80 (6): 896–901.
Yatich, Nelly J., Jiang Yi, Tsiri Agbenyega, Archer Turpin, Julian C. Rayner, Jonathan K. Stiles, William O. Ellis, et al. 2009. “Malaria and Intestinal Helminth Co-Infection Among Pregnant Women in Ghana: Prevalence and Risk Factors.” The American Journal of Tropical Medicine and Hygiene 80 (6): 896–901.
Zlotkin, Stanley, Samuel Newton, Ashley M. Aimone, Irene Azindow, Seeba Amenga-Etego, Kofi Tchum, Emmanuel Mahama, Kevin E. Thorpe, and Seth Owusu-Agyei. 2013. “Effect of Iron Fortification on Malaria Incidence in Infants and Young Children in Ghana: A Randomized Trial.” The Journal of the American Medical Association 310 (9): 938. doi:10.1001/jama.2013.277129.