On behalf of USAID, SPRING organized a Micronutrient Powders (MNP) Consultation on October 19–20, 2015 to summarize, review, and analyze real experiences in MNP programming for children 6–23 months of age. The three objectives of the MNP consultation were to (1) using existing documents, reports, and country experiences, identify and summarize experiences, with an emphasis on lessons learned from the field within MNP programming; (2) define essential logistic components that should be included in any MNP program to ensure national ownership, context specificity, and sustainability; and (3) prioritize an MNP operational research agenda.
There is limited published implementation research from country experiences on MNPs; therefore, it is important to garner lessons learned from countries currently implementing MNPs and to use the existing global guidance—World Health Organization, Home Fortification Technical Advisory Group resources—in planning for MNP programs. Twenty country- and global-level experts participated in one of the three working groups to review and summarize the current experiences in implementing MNPs. The working groups were divided by MNP programmatic area: Planning, Coordination, and Supplies; Delivery Platforms and Social and Behavior Change Communication (SBCC); and Monitoring and Supervision.
The MNP consultation was an opportunity for working group members to discuss critical lessons learned in operationalizing MNPs and learn about the knowledge and experience of participating country representatives. This report summarizes the preliminary findings on the key lessons learned, as identified by working group members, include the following:
- Planning, Coordination, and Supplies: Sensitize key stakeholders using efficacy data and economic return on investment data; ensure adequate technical (including local researchers) and financial resources; integrate planning at the onset with the aim of achieving scale and sustainability; and, coordinate through existing bodies that already have established trust and credibility. Ensuring reliable and consistent supplies requires carefully considering procurement options, which include importing finished product, locally packing imported bulk premix, and importing raw materials to mix and pack locally.
- Delivery Platforms and SBCC: Decision-making around, delivery platforms, and SBCC are highly dependent on context and need to take into account the infrastructure, capacity, and systems that currently exist in a given country; community-based platforms may be the most accessible for families as they are a well-established platform for health, nutrition, and infant and young child feeding (IYCF) interventions; and minimal behavioral objectives should focus on repeated and appropriate use of MNP and key related IYCF messages, especially around the timely introduction of complementary foods and ensuring optimal viscosity.
- Monitoring and Supervision: Factors for success appear to be around the alignment of capacities for monitoring and staff, streamlined and integrated systems, strong feedback loops for decision making, and programs founded on solid program theory. Further defining standard measures of success for MNP programs is needed, for example, developing coverage targets. Critical to sustaining an MNP program are setting up a training of trainers system to build capacity, refresher trainings, and supportive supervision. Scale-up hinges on funding, political will, adequate technical support, and strong local capacity.
Countries found that successful programs tend to build research into MNP programs at the onset and throughout the program to help inform implementation and adaptation. Operational research gaps at global and country level that were prioritized include: (1) a minimum package for delivering MNP programing including SBCC; (2) strategies and SBCC components to improve (repeat) coverage and intake adherence of MNPs; (3) the influence of MNP programs on IYCF practices and the influence of IYCF programs on MNP practices; (4) how context affects the choice of delivery platform, training, supportive supervision, and monitoring; and (5) how to make training of supervisors more effective—supervision and supportive supervision—especially in cases with high turnover.
Building on the findings and discussions during this MNP consultation, three white papers will be developed on planning, coordination and supplies; delivery platforms and SBCC; and monitoring and supervision, which will be published in a relevant journal during the coming year. In addition, USAID Missions are frequently discussing the appropriateness of including the use of MNPs in programs, and SPRING will develop an USAID Implementation Brief on MNP programming for country missions.
Background
Rationale for Consultation
Interventions to Deliver Micronutrients to Populations:
- Food Fortification: Addition of one or more essential nutrients to a foodstuff, regardless of whether the nutrient(s) was present in the food in its original form, with the intention of preventing inadequacy/deficiency of one or more nutrients at the population level.
- Mass fortification: The addition of micronutrients and minerals to edible products (staples, oil, sugar, milk, salt, etc.) that are widely consumed by the general population
- Targeted fortification: The addition of micronutrients to specific foods that are consumed by a specific population or age group, for example emergency rations for refugees or complementary foods for children in preschool feeding programs
- Commercial-driven fortification: the voluntary effort by food manufacturers to fortify their product with micronutrients to enhance marketability of their product
- Supplementation: Provision of vitamin and mineral supplements to targeted populations
- Point of use ‘fortification’: Consists of adding a mixture of micronutrients in powder form to semi-solid food.
- Traditional pharmaceutical preparations: Supplementation is targeted to vulnerable groups – such as iron folic acid for pregnant women, adolescent girls, and WRA; vitamin A for children under five.
Meeting the nutritional needs of young children is a particularly important issue in global health—insufficient nutrition during periods of rapid growth can have serious effects on future health and other outcomes (Bhutta 2008). The long-term solution for solving micronutrient inadequacy is to ensure a sustainable and diverse diet using food-based approaches. However, because of different ecological, economic, and cultural factors, many times it is necessary to complement the nutritional value of the diet using specific interventions, such as food fortification or supplementation (Bhutta 2013). There are several interventions that are used to deliver micronutrients to populations, including food fortification and supplementation (see inset). One promising supplementation strategy, when the coverage or efficiency of food fortification are limited, is the use of micronutrient powders (MNPs), which are mixed in the child’s food, in a strategy popularly called point-of-use fortification or home-fortification.
Evidence from efficacy studies has shown that MNPs improve iron status and reduce anemia (De-Regil et al. 2011) and as a result the World Health Organization (WHO) currently recommends providing MNPs, containing iron, zinc, and vitamin A, to children 6-23 months of age as a strategy to control anemia. As with any other iron-delivering strategy, WHO recommends provision of MNPs in conjunction with malaria control measures where relevant, a combination which has been shown to confer no additional malaria risk among MNP consumers (Zlotkin et al 2013). However, it is important to note that some studies have suggested other unintended adverse consequences of MNP provision to children, specifically around intestinal infections (Jaeggi et al. 2014), diarrhea, and respiratory morbidity (Soofi et al 2013). It should be noted that efficacy and safety of the use of iron is independent of the mechanism of delivery, and MNPs are only one delivery mechanism, around which this consultation focuses.
An active Home Fortification Technical Advisory Group (HF-TAG) created an extensive library of MNP guidance and tools; since 2009, they have had an active community of practice to address programmatic guidance around MNPs. As of 2011, projects/programs using MNP’s have been introduced in 22 countries, including small-scale pilots in 9 countries, sub-national projects in 21 countries, and national programs in 4 countries (UNICEF/CDC/HF-TAG 2011); by 2013, the number of countries where activities using MNP’s had doubled to 43, with additional projects planned in 21 new countries in 2014 (UNICEF 2013). Although most of these interventions are small in scope, MNPs reportedly are being delivered at scale in 13 countries (UNICEF 2013).
The U.S. Agency for International Development (USAID) Missions are frequently discussing the appropriateness of including MNP as a component of their programs; however, the focus to date has been mainly limited to procuring MNP sachets. To deliver high-quality programs, missions need up-to-date guidance on all aspects of programming for MNPs including planning, design, implementation, and monitoring, as well as the cost and training activities associated with each of these.
On behalf of USAID, the Strengthening Partnerships, Results, and Innovations in Nutrition Globally (SPRING) project organized an MNP consultation that would provide contextualized operational guidance to USAID missions, and which may also be useful for other institutions considering the use of MNPs in their projects. The aim of this consultation was to summarize, review, and analyze real experiences in MNP programming for children 6–23 months of age. The consultation did not include discussions about biological impact or formulation of MNPs; which, although very important, are topics more appropriate for scientific gatherings.
Objectives
The three objectives of the MNP consultation were to complete the following:
- Using existing documents, reports, and country experiences, identify and summarize experiences; emphasize lessons learned from the field within MNP programming.
- To ensure national ownership, context specificity, and sustainability, define essential logistic components that should be included in any MNP program.
- Prioritize an MNP operational research agenda.
Consultation Format
The consultation process began in July 2015, when 20 country- and global–level experts were invited to participate in one of three working groups; divided by programmatic areas—Planning, Coordination and Supplies; Delivery and Social and Behavior Change Communication (SBCC); and Monitoring and Supervision. The working groups were tasked with developing three white papers on these topics to meet the objectives stated above. Each working group, led by a chairperson, and assisted by a SPRING-staff rapporteur, met via teleconference for three months before the consultation meeting. During these teleconferences and via emails, working groups began preparing draft white papers to meet the consultation objectives.
The two-day face-to-face meeting—the subject of this report—was an opportunity for working group members to meet in person and develop their working papers. During the consultation, working groups deliberated within their respective groups to refine the white papers and share and discuss draft content with all the meeting participants. Additional presentations and panels were held during the meeting to highlight specific program challenges and contextual factors affecting the operationalization of MNPs.
Participation
A wide range of participants attended the two days of discussions and working sessions, including country-level programmers and policymakers from Bangladesh, Bolivia, Guatemala, Indonesia, Kyrgyzstan, Mongolia, and Tanzania; and global advisors from the Pan-American Health Organization (PAHO); Centers for Disease Control and Prevention (CDC); Global Alliance for Improved Nutrition (GAIN); Helen Keller International (HKI); Hospital for Sick Children; International Food Policy Research Institute (IFPRI); The Manoff Group, Inc.; PSI; Sprinkles Global Health Initiative (SGHI); Swiss Institute of Technology (EHT); Tufts University; United Nations Children’s Fund (UNICEF); and World Food Programme (WFP). A total of 46 members participated, including the USAID and SPRING staff.

Plenary Proceedings by Session
Inaugural Session:
The inaugural session’s objectives were to initiate and establish expectations for the MNP Consultation. Leaders of the convening organizations—Kellie Stewart (USAID), Sorrel Namaste (SPRING), Christina Nyhus Dhillon (SPRING consultant), and Omar Dary (USAID)—emphasized the importance of the consultative process, applying global expertise and country evidence to garner lessons learned; defining essential components of an MNP program; and identifying operational research gaps. The consultation deliberations will inform the development of the operational guidance on MNP programming for USAID Missions.
Setting the context of MNPs in public health nutrition programming
Omar Dary is a Senior Nutrition Advisor of the Bureau for Global Health at USAID. For the last 25 years, Dr. Dary has provided technical assistance to more than 40 countries in the areas of micronutrient interventions and nutrition surveillance.
To set the context of MNPs in public health nutrition programming, it is essential to understand the current limitations of developing countries to improve nutrition. Important aspects of public health nutrition programming include building national- and local-capacities, using an intersectoral approach; and, within the framework of implementation science, incorporating knowledge and experiences from the field that go beyond the published literature. The success of programs depends on context assessment to define needs and causes and determine the potential usefulness of strategies and interventions. Furthermore, as resources are finite, interventions must be selected wisely to maximize a country’s own investments.
There is proof of concept that MNPs could be a useful means to deliver micronutrients, and therefore able to prevent and correct nutritional anemia and iron deficiency in children when used as recommended. However, MNP policies and programs must take into account a combination of complementary strategies—including biofortification, food fortification, and traditional supplementation—which are different mechanisms to provide essential micronutrients. These interventions vary by cost, reach, and programmatic efficiencies—production, delivery, accessibility, and monitoring—and they depend on country context and the population in need. Although it is known that delivering essential micronutrients through MNPs is efficacious, less is known about the program effectiveness of MNPs. The focus of the consultation was to delve into components of program implementation; encompassing standards and regulations, production, supervision, and utilization.
View the presentation (PDF, 193 KB)
Session I: Summarizing the Global Context
The objectives of the session were to provide a technical background on MNP programming evidence, guidance, and experiences. The session was moderated by Sorrel Namaste, Technical Advisor for Anemia (SPRING) and included three presentations, followed by questions and answers (Q&A).
Overview of biological impacts of the use of MNPs: a focus on infants and iron
Michael Zimmerman is a professor at the Department of Health Sciences and Technology at the Swiss Federal Institute (ETH) Zurich, Switzerland; where he is head of the Laboratory for Human Nutrition. Dr. Zimmerman’s research field is human nutrition, with a focus on micronutrient deficiencies.
Michael Zimmerman presented an overview of the biological impacts when MNPs are used, focusing on infants and iron. MNPs were developed as an intervention to increase micronutrient intake, particularly iron, in children under 2 years. The Cochrane systematic review—which assessed the effects and safety of MNPs on nutrition, health, and developmental outcomes in children under 2 years—found that projects based on MNPs reduced anemia and iron deficiency in populations with different baseline anemia prevalences, at all ages, at all durations of intervention, and in both malaria-endemic and malaria sporadic settings (De-Regil et al, 2013). Nevertheless, following the Pemba study (Sazawal et al., 2006), there has been concern about the potential harm of routine iron supplementation to young children in areas of high malaria transmission. However, a recent study in Ghana (Zlotkin et al., 2013), a malaria-endemic setting, found that daily use of MNP, when accompanied with malaria prevention measures (insecticide-treated bed nets) and appropriate malaria treatment, did not result in an increased incidence of malaria among young children. Another systematic review (Salam et al. 2013) looking at the effectiveness of MNP in women and children found that, with use, children had a 4 percent increased risk of diarrhea. This effect was mainly noted in a study in Pakistan showing the increased risk of bloody diarrhea from iron in MNPs with or without zinc (Soofi 2013).
Iron bioavailability, uptake, and absorption are complex; and iron is a growth limiting nutrient for many pathogenic gut bacteria. This is an issue with MNPs, because the iron, which tends to be poorly absorbed, produces large increases in colonic iron. Iron may decrease the abundance of beneficial bifidobacteria; and increase potential pathogens like enterobacteria, shifting the gut microbiome in a way that increases gut inflammation and, possibly, diarrhea in infants. More research is needed to understand if changes in the gut translate into clinically relevant diarrhea or sepsis, and how iron can be more safely delivered to children.
Applicable learning for MNP operationalization
- Since the Cochrane review, several other studies in different countries have supported the findings that MNPs are efficacious vehicles to deliver micronutrients, and so to reduce anemia and iron deficiency in children 6–23 months of age.
- It is important to be aware of the potential negative side effects of iron-containing supplements targeted to children, like MNPs.
- MNP programs will need to consider diarrhea as a potential outcome and subsequent effect on adherence.
- The study raises an important question on how iron can be delivered more safely to infants using MNPs (or any other iron-delivering strategy). Potential solutions include the addition of a prebiotic or phytase in MNPs to safely administer iron and correct iron deficiency anemia; this is currently being explored by a study in Kenya.
View the presentation (PDF, 4.4 MB)
Programmatic experiences with MNPs
Ruth Situma is a nutrition specialist working on micronutrient programs at UNICEF headquarters. Ms. Situma has more than 15 years of work experience designing, implementing, and monitoring food and nutrition security programs in development and emergency contexts.
Ruth Situma presented an overview of programmatic experience with MNPs that covered the evolution of MNP programs. To present global progress, challenges, and the way forward, she included data from the UNICEF Supply Division and the 2011 UNICEF/CDC Global Assessment of Home Fortification (2013) and UNICEF Nutridash 2014 (not yet public). Although MNPs were invented and developed in 1995 as complementary manner to increase micronutrient intakes, and initially often used under emergency settings (Rah et al, 2012), WHO guidelines on MNPs for 6–23 month-old children (WHO 2011), and program guidance by the HF-TAG (HF-TAG 2010) were developed much later, in 2011 and 2010, respectively. From 2011 to 2013, there has been an impressive global scale up of MNP interventions—predominantly in the regions of Asia and Africa—increasing from 36 interventions in 22 countries to 62 interventions in 43 countries. In 2011, four countries were implementing at national scale, which increased to 13 by 2013. Countries have been steadily increasing orders for MNPs, with 50 percent of countries using local materials for packaging, and a difference in formulation by country—nine countries were using the five micronutrient formulation, while the others were using the 15 micronutrient formulation or another formulation. In 2013, the most common target groups were 6–23 months (44 percent) and 6–59 months (25 percent). The majority of countries are using a public distribution channel, but market-based approaches have been tried too.
MNP Summary of Progress by Region
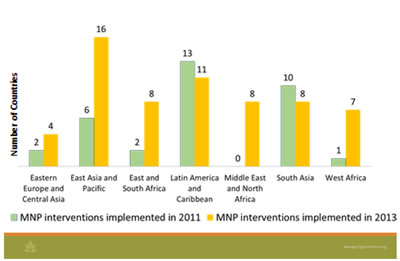
Applicable learning for MNP operationalization:
- Programs often only focus on the product—supply, procurement, etc.—without well-designed sustainable programs in place. More successful examples in Kyrgyzstan and Madagascar have put some essential programmatic components in place before starting the program.
- The majority of MNP programs are anchored in infant and young child feeding (IYCF) programs; however, many of these programs are weak and require significant effort to improve and sustain the program. Additionally, but less common, integration platforms are anemia/micronutrient, school feeding, and social protection programs.
- As programs are scaling up, to ensure quality programming in MNPs, increased technical support, tools, and resources are needed.
- HF-TAG—an important resource for growing capacity needs—includes a consolidated tool kit on best practices and examples of implementation MNPs, and an online community of practice for inter-country learning; they also present a series of webinars on MNP-relevant topics.
- Rapid growth in MNP programs is an excellent opportunity for inter-country and inter-agency learning.
- Countries need to design programs that are sustainable, catalyze partnerships and innovations for improved intervention quality, and generate information and knowledge to promote global learning.
View the presentation (PDF, 838 KB)
Existing Guidance around MNPs
Stanley Zlotkin is a professor of pediatrics, public health sciences, and nutritional sciences at the University of Toronto, a Senior Scientist at The Hospital for Sick Children Research Institute, and a member of the Department of Paediatrics at SickKids. Dr. Zlotkin led the development of micronutrient powders trademarked “Sprinkles.”
Stanley Zlotkin reviewed the existing resources to provide support for MNP programmers and the current guidance. Providing micronutrients in a powder form has been an innovative and efficacious way to deliver essential micronutrients to young children. The concept of MNPs is simple—“they deliver micronutrients in appropriate qualities and amounts, and therefore their use has been able to reduce anemia, they have been well-accepted because parents find them easy to use, and they can be produced in large quantities, at a low cost”. However, 17 years after their development, the challenge has been the delivery of MNPs— operationalizing MNPs is not simple.
MNP Evolution
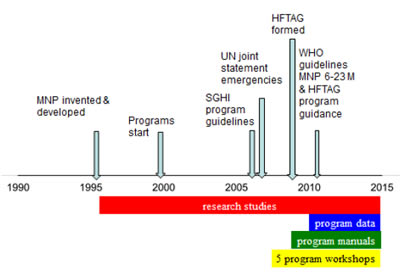
Applicable learning for MNP operationalization:
- A number of existing guidelines have been produced to help develop MNP programs.
- The 2011 WHO Guideline: Use of multiple micronutrient powders for home fortification of foods consumed by infants and young children 6-23 months of age recommends MNPs—with a minimum recommendation of 12.5 mg of elemental iron, preferably as encapsulated ferrous fumarate; 5 mg of elemental zinc; and 300 μg of retinol (vitamin A)—to improve iron status and reduce anemia for infants and children 6–23 months of age, in populations where the prevalence of anemia in children is more than 20 percent (WHO 2011).
- WHO Essential Nutrition Actions, which summarizes the guideline mentioned above, and which also recommends the evaluation of nutrition status in children under 5 years of age, adequate measures for management of malaria and diarrhea, and optimal IYCF practices as part of any program that considers the use of MNPs (WHO 2013).
- For manufacturing guidance, countries can use the HF-TAG Quality Manual on Micronutrient Powders—A Guiding Document, which describes the requirements for MNP operations for proper manufacturing practice; it focuses on product safety and, equally, on food and consumer safety, as well (HF-TAG, UNIDO 2014).
- For programmatic guidance, countries can use the Planning for Program Implementation of Home Fortification with Micronutrient Powders (MNP): A Step by Step Manual, which outlines the key steps and considerations that inform the design, implementation, and management of MNP programs (HF-TAG, 2015)
View the presentation (PDF, 735 KB)
The concept of MNPs is simple, but there is nothing simple about the operationalization of MNPs.
- Dr. Stanley Zlotkin
Session II: Brief of Countries’ Experiences
The objectives of the session were to provide, in plenary, findings from implementation research and programming experiences from country experts. Tim Quick, Senior Advisor for HIV & Nutrition (USAID), moderated the session; it which included two presentations and a panel of seven country implementers.
Micronutrient Powders Project: Key Findings 2013
Kanika Bahl is a principal and managing director at Results for Development Institute (R4D), where she established and leads the Market Dynamics practice. R4D’s Market Dynamics practice has developed strategies to ensure the sustainable availability of high-quality products to address malnutrition, AIDS, malaria, and neglected diseases.
Results for the Development Institute’s Market Dynamics Team undertook an evaluation, with support from the Bill & Melinda Gates Foundation, to identify the opportunities and feasibility of using market-based approaches to deliver MNPs. The report—published in 2013 and entitled Nutrition for a Better Tomorrow (Bahl et al, 2013)—was a landscape analysis of demand and supply side opportunities and challenges from experiences in six countries: Bangladesh, Bolivia, Ethiopia, India, Kenya, and South Africa. Kanika Bahl, the managing director for R4D, presented a brief summary of the key findings.
Applicable learning for MNP operationalization:
- MNPs are relatively expensive to produce and they have a very small profit margin; therefore, manufacturers do not have an incentive to produce MNPs, making the production landscape concentrated and limited. Private sector scale-up is not recommended.
- Private sector MNPs costs may be prohibitive for most consumers where MNPs are recommended; but, they may be offset by social protection programs (for example, Oportunidades program in Mexico).
- Although the public sector should be the primary distribution channel in most settings, socially orientated or commercial distribution can increase reach/coverage, especially among middle and high income consumers. For example, in Bolivia, where MNPs are a national program, roughly 10 percent of MNPs are delivered through the private sector.
View the presentation (PDF, 654 KB)
Delivery and Social Marketing Research
Ietje Reerink is currently working as a consultant for PSI's child survival department, based in Madagascar, and providing ongoing support to the planning, design, implementation, and evaluation of a pilot project for introducing a socially marketed MNP. She is also assisting UNICEF and PSI with preparing a summary document on multi-country experiences with the social marketing of MNPs.
PSI is currently conducting a learning study to examine the feasibility and sustainability of MNPs in four settings: Laos, Somaliland, Mozambique, and Madagascar. UNICEF is supporting the project to provide evidence on the potential for market-based approaches to optimize access and use of MNPs. Ietje Reerink, a PSI consultant leading this project, presented a brief summary of the current status and what they have learned, thus far, in the project.
Applicable learning for MNP operationalization:
- Most challenges to-date center on product quality, including taste, smell, and color changes, which have discouraged mothers from using MNPs. Food vehicles, can also be a barrier. In Laos, sticky rice is too thick for the addition of MNPs.
- Earlier and better collaboration with the Ministry of Health (MOH) are needed. In Mozambique, this may have helped avoid problems with the product branding, which had to be changed after the government classified MNPs as a food supplement.
- Messaging needs to be continuously updated. This is based on learning in Somaliland where MNPs are marketed through pharmacies; after three months, the focus had to shift from first-time users to continued users.
- Public sector delivery constraints—facility numbers, stockouts, etc.—limit the reach of MNPs; therefore, private-sector approaches, built off the public sector system, may increase access. The private sector can also focus on children 24–59 months of age, outside the most vulnerable target group of 6–23 months, who the public sector more effectively reaches.
View the presentation (PDF, 1.2 MB)
Lessons learned:
- By 2012, after 15 years of implementation in the country, funding is the greatest challenge.
- Program stability is also threatened by the limited and changing political will, high staff turnover, and poor monitoring and supervision. Areas handed over to the government for implementation lack sufficient monitoring; coverage is, therefore, poor.
Country Panel
To enrich discussions and cross-learning, seven country-level implementers of MNP programs were invited to participate in a panel to answer the following questions:
Lessons learned:
- Challenges with MNP implementation include the need for significant interpersonal communication with mothers; especially because immediate results are rarely seen from taking MNPs.
- After it was offered, caregivers more readily accepted a flexible dose (2-3 times a week from age 6 to 23 months) compared to 6 month cycles where MNPs are taken daily for 2-3 months and stopped for 2-3 months before resuming.
- Maintaining sufficient supplies to respond to demand remains a challenge. Only one manufacturer provides MNPs in Bangladesh.
- Local pediatricians can be a barrier to uptake because they may not support the use of MNPs and focus on counseling mothers on dietary changes only.
- It is still unclear if program sustainability can be ensured.
- KEYS TO SUCCESS: What steps did you take that you thought were essential to the success of your MNP program?
- LESSONS LEARNED: Were there any unexpected challenges you encountered in implementing an MNP program and what would you do differently if you were to start over?
Applicable learning for MNP operationalization:
Mongolia
Dr. Narantsetseg Tsevegsuren, the representative from Mongolia, has served as the health national coordinator for the last seven years with World Vision International Mongolia providing technical support and strategic direction to the organization’s health program activities throughout the country.
Keys to success included having a secure funding stream, government approval, and free distribution. Positive pilot results also helped build steady scale-up. The distribution approach, established through the government supply systems and implemented through the community-based government system—with trained health facilitators who supervise and monitor health volunteers—has worked well.
Bangladesh
Dr. Mohammad Raisul Haque serves as program head of BRAC’s nutrition programs, leading the implementation team with operational and technical guidance in 243 sub-districts, covering a population of 62 million in Bangladesh.
Keys to success included coaching and demonstrations that were built into the community volunteer approach—which resulted in a rapid increase in the sale of MNPs—from 10,000 sachets in 2010 to 2 million sachets1 currently—and the ability to show reductions in anemia, which led to more political will and scaling up of the program.
Indonesia
Lessons learned:
- Poor product quality (bad smell) halted a planned scale-up in six districts in 2010. No systematic quality assurance is in place and program delays led to expired products. Supply storage and handling continues to be problematic.
- Planning was considered a challenge despite a number of planning committees.
Dr. Minarto Noto Sudardjo is the director of the Community Based Health and Nutrition Project, MCA-Indonesia since 2013; he has been involved in developing the nutrition program in Indonesia. Previously, he was the Director of Nutrition for the Department of Health.
Keys to success included having MNPs highly integrated with IYCF (including curriculum) and WASH programs, at all levels; building health worker capacity; developing a solid SBCC campaign—including cooking demonstrations—close monitoring of the program, and using supervision checklists.
Guatemala
Ms. Claudia Roca is the resident advisor with the USAID | DELIVER PROJECT at John Snow, Inc. (JSI) in Guatemala. She leads the coordination of the project’s technical and administrative activities, and provides technical assistance to the key public health supply chain stakeholders.
The keys to success included having MNPs as part of the standard of care (free for children) and being incorporated into the government’s Zero Hunger strategy, which includes the development of culturally adapted information, education, and communication (IEC) materials into seven different Mayan languages. Furthermore, the MOH provided continuous health worker training and placed additional staff (nutritionists and educators) in regions with the largest number of children suffering from malnutrition. The health authorities had a high level of commitment, including implementation (national and regional) and budget support (national).
Lessons learned:
- Program challenges have primarily centered on supplies. A change from local to international procurements in 2012 to save costs resulted in less frequent (annual) procurements because of the long clearance processes—this led to overestimated forecasts, and loss of product after the best-date-to use.
- The internal supply distribution network is inadequate. Product loss is ongoing in health facilities with less than ideal storage conditions, where high temperatures and humid conditions alter the product.
- Currently, only delivery and coverage is monitored, but not uptake and adherence.
- Linking MNPs with vaccinations helped boost support among mothers. This was especially true after an MNP brand change led to confusion among mothers and, thus, lowered uptake.
Tanzania
Dr. Generose Mulokozi, currently a health scientist for the Tanzania Food and Nutrition Center (TFNC), served for three years as the public health nutritionist for an Abt Associate’s-managed USAID Feed the Future project in Tanzania. Through a commercial channel, Dr. Mulokozi pioneered the introduction of MNPs in eight districts of Tanzania mainland and Zanzibar.
Keys to success included advocacy with key stakeholders at the national level, discussions on the formulation of product from the onset, development of national MNP implementation guidelines, and collaboration with district authorities. Securing a regular tax exemption for MNPs allowed for a lower price point for the product. Further, SBCC activities for MNPs were implemented during growth monitoring, a critical contact point with caregivers. Training of gatekeepers at the community level allowed for dispelling myths around MNPs; multiple IEC materials for mothers/caregivers, as well as the general public, were important for awareness.
Lessons learned:
- Local Code of Marketing Breastmilk Substitutes regulations meant that MNPs could not be promoted or advertised; but, after much advocacy, the MNPs were exempted from this restriction.
- Later, the MOH stopped commercial distribution of MNPs because local health policy meant that children under five were entitled to free health care. After reclassifying MNPs as a food supplement, instead of a medicine, they were able to sell MNPs on the market.
- The district authorities should have done the formative research to inform the SBCC strategies. The mothers needed more training and counseling.
Kyrgyzstan
Lessons learned:
- An 18-month shelf life on the MNP product leaves little time for procuring, packaging, shipping and then delivering the product to the end user.
- Ensure that the platform of delivery is strong before it is used to deliver MNPs. Kyrgyzstan linked it to the Integrated Management of Childhood Illness (IMCI) and, thus, acquired all the weak components of that program, including the monitoring.
- It’s best to start social marketing right away at the beginning of a program, and ensure it is strong.
Ms. Cholpon Imanalieva has led UNICEF’s National MNP program in Kyrgyzstan since 2007, contributing to the design planning, piloting, and scaling up of program and monitoring of interventions. She is also one of the facilitators of the HF-TAG global Internet-based platform.
Keys to success included having a strong monitoring, research, and evaluation component because of the CDC involvement and capacity building, integration of MNPs into the existing early childhood development (ECD) program, and use of the Scaling Up Nutrition (SUN) platform to bolster support.
Bolivia
Lessons learned:
- Long-term sustainability will also depend on the municipal (local) financing in the new era of decentralization.
- Since 2007, local suppliers have been allowed to produce MNPs. The country policy was to produce MNPs nationally instead of importing them. The MOH monitors good manufacturing processes; they can require companies to produce MNPs when needed.
Dr. Gustavo Iván Tapia Terán is a public health specialist, and health and nutrition consultant in Bolivia. During the past 11 years, Dr. Tapia worked for nongovernmental organizations (NGOs) and international cooperation agencies—Plan International and UNICEF—and, in particular, consulted with UNICEF on their MNP program.
Keys to success included using the existing policies and frameworks to build sustainability into the MNP program, including the Zero Malnutrition initiative; which had both technical and financial backing. MNPs were also articulated as more than a single strategy, but part of a larger package of programs; including Mi Salud and preventive programs, such as immunization. Regular meetings of a national health information committee were held, which maintained the program’s sustainability.
Integration is the key to success for MNP programming.
Session III and IV: Reports of the Working Groups
The objectives of session III were to deliberate as a working group on their designated topic: Planning, Coordination and Supplies; Delivery and SBCC; or Monitoring and Supervision. During session IV, each working group reported back and, for feedback, discussed their current thinking on each topic with the plenary. Christina Nyhus Dhillon, consultation focal point (SPRING consultant), moderated the session.
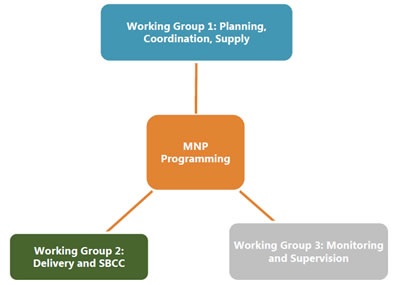
Working Group 1: Planning, Coordination, and Supplies
Rolf Klemm, the Vice President for Nutrition at HKI, is also a senior associate in the Program for Human Nutrition at the Johns Hopkins University Bloomberg School of Public Health. Dr. Klemm is the chairperson for working group 1.
The group’s objectives were to review and summarize experiences around the lessons learned when they planned the coordination of MNP programs, including national-level policy decisions and planning, purchasing/production, and standards and regulations. Rolf Klemm, the chairperson; and three members—Ruth Situma, Claudia Schauer, and Nigel Sunley—presented experiences with these topics, one person for each sub-topic. Because limited literature is available for this phase of MNP programming, most of the findings are from interviews with implementers in-country. The group is collecting a wide range of experiences; they plan to offer case studies of successful countries and factors of roll out and scale-up.

Key findings thus far:
In summary, to create an enabling environment and to sensitize stakeholders, programs have used prevalence and efficacy data, the novelty of prevention and improving complementary foods, and the similarity to food fortification. Also crucial are the return on investment (Copenhagen consensus), confidence in the messenger (e.g., local scientists), regional workshops, pilots (Nepal, Nigeria, Kyrgyzstan, and Cambodia), assurance of an initial donor-supported supply, and the WHO and HF-TAG guidelines and tools. Challenges may include quality issues with MNPs, side effects (diarrhea or dark stools), introduction during an unfavorable season (rainy), potential safety issues, confusion about the Code of Marketing of Breastmilk Substitutes (BMS), and the temporal nature of funding (e.g., when the pilot ends). Regulation plays an important role in the labeling and marketing, taxes, who can deliver, and import regulations.
- Feedback from plenary:
- A public-sector nutrition focal person is important for moving forward implementation of MNPs in country.
- For justification of using MNPs, include data on nutrient gaps in complementary foods.
- Significant issues exist around the causes of anemia and whether MNPs can address the main causes of anemia in a given setting (e.g., genetic hemoglobinopathies).
- Underlying nutrition issues and the number of other micronutrient programs vary greatly by country; for example, a concern around over-provision in Latin America is more of an issue when compared to Africa.
National-level planning and coordination, is best done through existing bodies that already have some level of trust and credibility. A group working on finance and policy, and another on technical issues, should coordinate their work. MNPs are often initiated in a donor-funded pilot, with the expectation that MNPs will ultimately be socially marketed—this may not be a reasonable strategy as governments may not be ready to assume the costs. Even if, in the beginning, they are funded through donor funds and in-kind contributions; national budgets need to contribute to funding scaled-up MNP programs. For acceptability, cost savings (in training and supervision), and sustainability, MNPs are best integrated into national guidelines, existing government supply chain, ECD, IYCF, or other existing programs—social protection insurance, vaccination, or outreach—but these programs are often weak.
- Feedback from plenary:
- Need to plan 5–10 years into the future and phase out the pilot approach.
- Examine countries using the private sector approach to determine if they are improving uptake/use and visibility.
- Noted that only five countries—Bolivia, Mexico, Brazil, Guatemala, and Indonesia—of the 13 national scale programs are government funded.
- To avoid resistance later, make the how of integration explicit from the onset.
- Even if supply is through commercial channels, the government can create demand and sensitize the population.
- MNPs have also been integrated into national food fortification committee which typically has strong cross-sector coordination.
The first step to ensuring reliable national and district supply is structured decision-making for obtaining MNPs. There are three sourcing options—importing the finished product, locally packing imported bulk premix, and importing raw materials (compounds that provide the micronutrients) to mix and pack locally—all require proper import, appropriate storage and supply chain, transportation, and distribution infrastructure considerations, as well as agreements on consequences for unacceptable product. (1) Importing the finished product offers quality assurance, but there are costs (import duties) and political considerations (countries receiving the manufacturing business). Importing the finished product also requires a supplier—long-term agreements can help—who will meet requirements on branding, labeling with local language, best-before dates, and traceability codes; and health claims based on the Code for the Marketing of BMS, which are also applicable to the other sourcing options. (2) Packing of bulk imported premix, requires an appropriate supplier, quality, and appropriate storage of the premix, context-appropriate packing materials and equipment, and quality control (seal is critical). (3) Importing raw materials and then mixing and packing locally, requires suppliers to provide the appropriate form of iron, other vitamins and minerals, and other materials, quality and storage of the materials (as in second option), appropriate packing materials and equipment (including mixer), and ensuring (through laboratory analysis) the composition and packing of the final product. Quality is not always better with an international supplier; a national supplier does not always have a better price or timeliness.

- Feedback from plenary:
- Consider a national standards logo to build consumer trust—found to be important in Tanzania, for example.
- Product quality assurance systems are needed all the way to the level of the consumer—see upcoming PSI manual that addresses this.
- Consider factors like environment—e.g., rainy season, heat—to the storage and transportation risks; national businesses need to understand that they may not make a profit—sustainability becomes an issue.
Working Group 2: Delivery and SBCC
Rahul Rawat is senior research fellow at IFPRI, based in Dakar, Senegal. At IFPRI, Dr. Rawat coordinates a portfolio of applied research focused on interventions delivered through a variety of delivery platforms to address maternal and child undernutrition. Dr. Rawat is the chairperson for working group 2.
The group’s objective was to review programming experiences when distributing MNPs using different delivery platforms: to outline key SBCC approaches in MNP programming and to identify key components for training and supervision related to MNP program implementation. The group found that, in addition to published literature, rich experiences from countries must be extracted to provide information and lessons learned in these topic areas. Further, these experiences must narrow in from delivery and SBCC for broader nutrition programming to highlight lessons learned that are unique to delivery and SBCC for MNP programming.

Key findings thus far:
In summary, delivery platforms are highly contextually specific. Countries implementing MNPs are using both public- and private-sector channels for MNP distribution; and, through the public sector, use a wide variety of program platforms. Based on initial explorations, community-based platforms may be the most accessible for families—they are a well-established platform for health, nutrition, and IYCF interventions. There is an established trust within the community; however, the frequency and quality of contacts is a critical factor for success. Potential case studies will be drawn from the following selection: Indonesia (community-based platform); Kyrgyzstan (facility-based platform); Bangladesh (mixed community- and market-based platform); Bolivia and Nepal (mixed community- and facility-based platform); Dominican Republic and Mexico (social protection platform); and Madagascar (market-based platform).
- Feedback from plenary:
- Consider how blanket distribution during emergencies can undermine an existing market approach.
- Look at Tanzania’s Abt-implemented market-based through the health facility approach, as well as early childhood development (ECD) programs.
- Give further consideration to children 12–23 months old, who may not be routinely receiving services.
- Further examine market-based approaches to determine if they are fulfilling the objective of reaching children 6–23 months old.
Integration of MNPs into programs, UNICEF Nutridash 2014
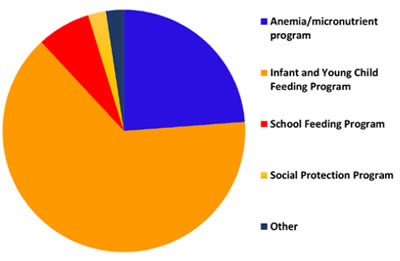
Many key components of training are generic for public health nutrition programs; however, the focus should be more than knowledge and should include counseling and problem-solving skills, particularly as families using MNPs may return with side effects. Further training should position why MNPs are important, and be fully incorporated into the delivery platform and related SBCC. Refresher training and supportive supervision are critical to sustaining the program.
- Feedback from plenary:
- Training of trainers is essential, because local trainers in the community can support the sustainability of the program.
SBCC related to MNP programs is very similar to SBCC in other nutrition program; however, it is important to focus on SBCC components that are unique to MNPs; SBCC should be incorporated throughout the project life cycle and used to make adaptations to the program. SBCC should be targeted to multiple audiences, including government officials, donors, influential community leaders, beneficiaries, and delivery staff. Key minimal behavioral objectives identified were the repeated use of MNP and appropriate use. Key related IYCF messages are the timely introduction of complementary foods and ensuring optimal viscosity. Critical elements that should be considered are children’s rejection of foods and strategies to cope, managing side effects, home reminders and prompts to sustain uptake, MNP sachet and cooking demonstrations, and clear pictorial directions on and inside the box.
- Feedback from plenary:
- SBCC should also target health facility staff, influential people (gatekeepers), and faith-based organizations.
- Dosing and varying regimens for MNPs is a challenge for messaging and caregivers’ perceptions. The dosing regimen in the WHO guidelines—take MNP for two months, stop for four months, and start again for two months—is based on randomized controlled trials, and/or funding limitations. Mothers find the instructions confusing. Many countries are moving to flexible dosing of every other day or three–four times a week—this schedule also has implications for messaging and it must consider the caregiver’s needs and perceptions.
Working Group 3: Setting the foundation for effective program delivery: Capacities and systems needed for monitoring, supervision, and continual improvement of micronutrient powder programs.
Lynnette M. Neufeld, Director of Monitoring, Learning, and Research at GAIN; leads a team dedicated to the strategic collection, translation, and use of evidence to guide the design and improvement of global nutrition programs. Dr. Neufeld is Chair of the Steering Committee of the Micronutrient Forum and is a council member of the International Union of Nutritional Sciences (IUNS). Dr. Neufeld is the co-chair of working group 3, with Alison Tumilowicz, Senior Technical Specialist in Monitoring, Learning, and Research at GAIN.
The group’s objective was to review the experiences of how MNP programs undertook monitoring, and formative process evaluation and supervision; and the extent to which this information has been used for continual program improvement, relying on published data and unpublished programmatic experiences. The group found that despite the need for continuous program improvement, systematic data collection and/or use of data for program improvement is generally not used and only the implementers have this information.

Key findings thus far:
In summary, some of the key factors influencing monitoring system designs include scale, budget, and delivery platform. Monitoring experiences will be drawn and summarized from Tanzania, Mongolia, Bangladesh, Kyrgyzstan, Indonesia, and Peru; which offer a variation of contexts, approaches, and outcomes. Documented process evaluations tend to come from pilots and not scaled-up programs. Factors for success appear to be around the alignment of capacities for monitoring and staff, streamlined and integrated systems, strong links to decision making, and founded on solid program theory.
- Feedback from plenary:
- Consider WFP’s work with strengthening MNPs within the national monitoring systems in the Dominican Republic.
- Qualitative data collection is often missing in process evaluations.
- Consider the use of a standard definition of measures of success for MNP programming—e.g. >80 percent coverage vitamin A supplementation programs—or whether the definition should vary by context.
Experiences around supervision are less frequently documented compared to monitoring systems. Challenges of supervision tend to be more systemic and not necessarily related to MNP programs. The group is still looking for good case studies for MNP supervision experiences. This area has less information and should be a focus for operations research. Linked to and following feedback from monitoring systems, continuous quality improvement (QI) is often not part of program cycles, at least in real life. Bangladesh may offer a good example of a functional quality improvement system. Operations research on the best ways to link monitoring to program improvement was identified as a need.
- Feedback from plenary:
- Consider Madagascar’s supervision experiences.
- Malawi has good examples of QI in a related program (SQ-LNS), which may be useful; as well as Bolivia’s experience using Lot Quality Assurance Sampling (LQAS).
Findings around scale and sustainability must first be better defined—what is scale in a targeted programming context? However, general challenges to scale often hinge around funding. Mongolia, Tanzania, Peru, and Guatemala may be good case studies for a range of sustainability and scale experiences (positive and less so). Factors for success tend to include advocacy, clear policies to support MNPs across multiple sectors, budgets, and dynamic programming. More generally, successful programs tend to build in research, have adequate technical and financial support, build the capacity of local experts, and are incorporated into the existing systems.
- Feedback from plenary:
- Consider how to maintain intense technical support as the program scales up.
Closing
In closing, Dr. Nyhus Dhillon discussed the next steps for the working groups: SPRING staff is expected to support working group teleconferences, literature reviews, circulation and compilation of the questionnaires, and key informant interviews. The aim is to compile the three working group papers into a supplement, unified by a SPRING-led introductory paper on the consultation and methodology, and submitted to a peer-reviewed journal.
Session V: Topics for the future
The objectives of the session were to identify research gaps and priorities for operational research through a facilitated discussion, and learning from ongoing research and programs around Small Quantity Lipid Nutrient Supplement (SQ-LNS), and Tufts University’s Food Aid Quality Review. Gwyneth Cotes, Director of Global Initiatives (SPRING), moderated the session; which included three presentations, followed by a Q and A.
Evidence and Programmatic Considerations for the Use of Small Quantity-Lipid-based Nutrient Supplements: Highlights from October 14–16 Consultation in Washington, DC
Zeina Maalouf-Manasseh is currently a research associate for nutrition with the USAID-funded Food and Nutrition Technical Assistance III Project (FANTA)/FHI 360 project; she manages a portfolio of nutrition and food security-related research.
Forty-two researchers, programmers, producers, and donors met during the week prior to the consultation to (1) share available efficacy and effectiveness evidence on using Small Quantity-Lipid-based Nutrient Supplements (SQ-LNS) to prevent malnutrition in programmatic settings; (2) discuss and summarize experiences on key operational topics, including challenges and lessons learned; and (3) outline the key operational conditions needed to roll out programs using SQ-LNS and to identify an implementation research agenda.
The meeting focused more on the evidence around growth—linear, and head circumference—instead of the impact on micronutrient deficiencies. Preliminary and mixed results were presented, but with minimal conclusive results to-date. Discussions around operationalizing SQ-LNS were limited to half a day and focused primarily around use and acceptability of these products.
Implications for MNP operationalization:
- Participants stated that packaging—type, size, design, and label messages and claims—is important: they recommended measuring adherence; looking at enhancers and barriers, and behavior change communications about adherence; intake mode and frequency; and potential undesirable effects.
- Participants also highlighted the importance of integration into IYCF and multisectoral programs; for example, using SQ-LNS as incentives for participation and ensuring that SQ-LNS consumption does not impact breastmilk intake, dietary diversity, and intake of other foods.
- Discussions included the mechanics around production (local production, cost, quality control, and inspection), logistics (classification of SQ-LNS in shipping and customs documents, storage and shelf life, and transportation, especially during the last mile), and distribution (market models and distribution channels, private sector approaches, government financing, and workloads in the health system).
- Local producers’ may have little experience with market models and consumers’ willingness to pay, among other things. Some very preliminary cost-effectiveness results on SQ-LNS programs were discussed.
- Materials and research studies from the International Lipid-Based Nutrient Supplements Project (iLiNS) project, which is ending, will be housed on the HF-TAG website.
View the presentation (PDF, 925 KB)
Research Engagement on Food Innovation for Nutritional Effectiveness Project/Food Quality Review Tufts University
Irwin Rosenberg is the Jean Mayer University Professor at Tufts and the Friedman School. Professor Rosenberg has been involved in nutrition and food policy issues, ranging from dietary guidelines and reference intakes to international nutrition recommendations for the elderly.
The Food Aid Quality Review (FAQR)—a Tufts University research program, supported by USAID—addresses food-based approaches for treating wasting, particularly moderate acute malnutrition (MAM). In phase I, the program produced a report on recommendations for improving the quality of and making changes to the U.S. food aid products and programs. In phase II, which is ongoing, the recommendations are being implemented in three countries—Burkina Faso, Malawi, and Sierra Leone—where cost effectiveness, safety, quality assurance, and harmonization with donors and agencies are being documented: Research Engagement on Food Innovation for Nutritional Effectiveness (REFINE) is a web-based repository for existing literature and ongoing research in food aid interventions and is part of the FAQR program. It aims to facilitate the exchange of knowledge among researchers and policymakers, encourages rigorous operational and policy-relevant research, and promotes a focus for future research investments that address research gaps. Research gaps are identified from consultations with stakeholders, literature reviews, and a systematic analysis of high-level policy guidance documents.
Implications for MNP operationalization:
- The process of improving the quality of food aid products and programs, and operationalizing them in different country contexts, is similar to MNP programs; lessons learned can be collected.
- Fourteen countries have ongoing studies using MNPs. Several MNP studies address six of the eight research gaps identified by REFINE, including the ideal composition of supplementary foods, acceptability of supplementary foods, best use of supplementary foods, efficacy of different supplementary food compositions, effectiveness of different supplementary food compositions, cost effectiveness of different supplementary food compositions, effective innovations of products and programming, and improvement of research communication. These studies are a resource for MNP researchers and programmers.
View the presentation (PDF, 441 KB)
MNP Operational Research Agenda
Maria Elena Jefferds is a behavioral scientist at the US Centers for Disease Control and Prevention (CDC); she has expertise in surveillance, monitoring, and evaluation of micronutrient programs; including the design and implementation of MNP programs.
The third objective of the MNP consultative process was to identify research gaps and prioritize an operational research agenda. The plenary explored the existing research gaps around MNP programming, and they started to prioritize topics for implementation research. The term implementation research is used broadly—the group brought up various definitions. They agreed that implementation research does not concern efficacy, or contribute to the evidence base or new findings in science. It is not defined by a methodology, but it is program focused and based on the needs and goals of the program. The broad goal of operational research is to translate findings of the research into improved operationalization of programs.
The plenary developed a list of 15 research questions (see annex III) that touch on a variety of topics around MNPs. The plenary agreed the implementation guidance developed from the consultation should have a global focus and should not be country specific. It might be useful to include guidance for the extremes—high and low resource constrained environments—and something midway, teasing out minimum standards and optimal standards. Further, the guidance would point to the importance of context in selecting delivery platforms.
The plenary prioritized five areas for operational research:
- Minimum package for delivering MNP programing, including SBCC
- Strategies and SBCC components to improve (repeat) coverage and intake adherence of MNPs
- Influence of MNP programs on IYCF practices and the influence of IYCF programs on MNP practices
- How context affects the choice of delivery platform, training, supportive supervision, and monitoring; including guidance for high-constrained versus low-constrained environments
- How to make training of supervisors more effective (supervision and supportive supervision), especially with high turnover.
Closing of Meeting
Gwyneth Cotes (SPRING) and Omar Dary (USAID) provided the concluding remarks; they expressed their gratitude to the participants for their time and contributions. Dr. Dary offered concluding thoughts that summarized the key points he observed during the two days of discussions:
- It is important to incorporate experiences from national-level programs into the white papers.
- Many elements contribute to successfully sustained programs and national ownership—a commitment beyond a financial commitment is necessary.
- MNP, as a product, still needs to be optimized, because many countries have had sub-standard products. Manufacturers must be convinced that if they invest in and offer a good product, the demand will be sustained.
- Product production needs a strong innovation—-it does not make sense that 90 percent of the costs of the sachets are the physical sachet and does not represent the cost of the micronutrients that are inside.
- To avoid regulatory problems, reduce taxes (and thus costs) and promote systems integration; we may want to consider including MNPs to be on essential drug lists.
- Adherence to MNPs may be problematic, which may mean that full reliance on a market delivery system could be ineffective.
- Some health systems issues are larger than the MNP programs—they limit program success and should also be improved: supervision, storage and distribution systems, governance, etc.
- Regular and refresher training are essential for high-quality programs; they should be strongly emphasized.
Ms. Cotes discussed the expected outcomes from the MNP consultation. In 2016, the working group white papers (Planning, Coordination, and Supplies; Delivery Platforms and SBCC; and Monitoring, Supervision and Continual Improvement for MNP programs) will be published; and a USAID Implementation Guidance Brief on the use of MNPs targeted to missions will be disseminated.
Conclusions
MNPs have been used as an efficacious delivery platform of micronutrients and, when used as recommended, they have reduced anemia in children 6–23 months. Countries should be aware of emerging safety concerns related to iron-supplementation in pathogenic environments.
Challenges remain in operationalizing and delivering MNPs that are very context-specific, and success of programs that include the use of MNPs depend on them.
Because there is little published implementation research on MNPs, it is important to examine and document the lessons learned from countries currently implementing MNPs for wider dissemination and continue to promote implementation research in this area.
The main challenges identified by countries represented at the Consultation include:
- Many issues with MNP programs are due to larger issues in the health systems and therefore limit program and success and need to be improved overall.
- Issues with maintaining a reliable and sustainable supply, quality of product, and product loss due to 18 month shelf life and sometimes inaccurate forecasts are still stalling the effective implementation of MNP programs.
- Decision-making around classification of MNP as a drug or food supplement and associated regulations needs to be informed by the particular context and legal environment.
- Although integration of MNP into an existing delivery platform (e.g. IYCF) is ideal; however, it is challenging if the platform is already weak.
- Significant sensitization, awareness building, and interpersonal communications are needed to ensure coverage and adherence to MNPs, which is very time and resource intensive.
- Monitoring is often weak, and at best only covers delivery and coverage, rarely adherence.
The main success factors identified by countries represented at the Consultation include:
- Integration at all levels— into national policy/frameworks and existing government systems/platforms— is critical and a key to success.
- Secure funding and robust technical support were essential for success and sustainability.
- A solid SBCC campaign which is culturally adapted to context with a demonstration focus is imperative.
- A strong monitoring, research, and evaluation component is vital for program improvement and sustainability.
- Regular meetings and communications with stakeholders is crucial for sustainability.
Results of the Consultation
- All participants received updates on global-level programmatic and scientific guidance around MNPs.
- New learning from the field was shared and discussed in presentations, panels, and Q&A sessions.
- Working groups had active participation and discussions. Members shared and began to document some of the critical lessons learned in the various contexts of MNP programs, across multiple countries.
- Working groups began to define some areas of consensus around good practices in MNP programming—i.e., flexible dosing regimens, continuous SBCC strategies, national ownership—and identified some countries for case studies to highlight certain areas of learning.
- After two days of deliberations, some of the emerging operational research needs in MNP programming were identified and some basic prioritization was completed.
- Working groups were able to complete more work on their white paper outline and content, and solidify some of the next steps towards completing the white papers, including methodological approaches.
Footnotes
1 This amount would be sufficient to cover 16,667 children (using 60 sachets every 180 days) per year.
References
Bahl K., E. Toro, C. Qureshi, P. Shaw. Nutrition for a Better Tomorrow: Scaling Up Delivery of Micronutrient Powders for Infants and Young Children. Results for Development Institute. Washington, D.C. 2013. http://resultsfordevelopment.org/sites/resultsfordevelopment.org/files/resources/Nutrition-for-a-Better-Tomorrow-Full-Report_0.pdf
Bhutta, Z.A. 2008. “Micronutrient needs of malnourished children.” Curr Opin Clin Nutr Metab Care. 2008 May; 11(3):309–14.
Bhutta, Z.A., J.K. Das, A Rizvi, M.F. Gaffey, N. Walker, S. Horton, P. Webb, A. Lartey, and R.E. Black. 2013. “Evidence-Based Interventions for Improvement of Maternal and Child Nutrition: What Can Be Done and at What Cost?” The Lancet 382 (9890): 452–77.
De-Regil, L.M., P.S. Suchdev, G.E. Vist, S. Walleser, and J.P. Peña-Rosas. 2013. “Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age.” (Review) Evid.-Based Child Health. Cochrane Review Journal 2013 8:1: 112–201.
HF-TAG 2010. Home Fortification Technical Advisory Group (HF-TAG) website; Global Alliance for Improved Nutrition (GAIN). Geneva, Switzerland, 2010;
HF-TAG, UNIDO 2014. HF-TAG Quality Manual on Micronutrient Powders—A Guiding Document. http://www.hftag.org/resource/hf-tag-quality-manual-on-micronutrient-powders-a-guiding-document/
HF-TAG. 2015. Planning for Program Implementation of Home Fortification with Micronutrient Powders (MNP): A Step by Step Manual. http://www.hftag.org/resource/hf-tag-planning-for-implementation-manual-may-2015-pdf/
Jaeggi, T., G.A. Kortman, D. Moretti, C. Chassard, P. Holding, A. Dostal, J. Boekhorst, H.M. Timmerman, D.W. Swinkels, H. Tjalsma, J. Njenga, A. Mwangi, J. Kvalsvig, C. Lacroix, and M.B. Zimmermann. 2014. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Bethesda, Md.: National Center for Biotechnology Information, U.S. National Library of Medicine. http://www.ncbi.nlm.nih.gov/pubmed/25143342 (accessed on 11/30/2015)
Salam, R.A., C. MacPhail, J.K. Das, and Z.A. Bhutta. 2013. “Effectiveness of Micronutrient Powders on Women and Children.” BMC Public Health. 2013 13(Suppl 3):S22. http://www.biomedcentral.com/1471-2458/13/S3/S22
Sazawal, S., R.E Black, M. Ramsan, H.M Chwaya, R.J. Stoltzfus, A. Dutta, U. Dhingra, I. Kabole, S. Deb, M.K. Othman, and F.M. Kabole. “Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomized, placebo-controlled trial.” Lancet 2006, Jan 14; 367 (9505):133-43.
Soofi, S., S. Cousens, SP Iqbal, T. Akhund, J. Khan, I. Ahmed, A.K. Zaidi, Z.A. Bhutta. “Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial” Lancet 2013 Volume 38, Issue 9886, 29-40.
United Nation’s Children Fund (UNICEF). 2014. Nutridash 2013 – Global Report of the Pilot Year. New York: UNICEF. http://www.sightandlife.org/fileadmin/data/News/2015/2_Feb/UNICEF_Global_NutriDash_report_2013.pdf (accessed on 2/15/2016)
United Nation’s Children Fund (UNICEF)/Centers for Disease Control and Prevention (CDC)/Home Fortification Technical Advisory Group (HF-TAG). 2011. Global Assessment Report 2011- Data on MNPs. Geneva: HF-TAG. http://www.hftag.org/resource/global-assessment-of-home-fortification-interventions-2011-pdf/
World Health Organization (WHO), World Food Programme (WFP), and United Nation’s Children Fund (UNICEF). 2007. Preventing and controlling micronutrient deficiencies in populations affected by an emergency: Joint statement by WHO, WFP, and UNICEF. http://www.who.int/nutrition/publications/WHO_WFP_UNICEFstatement.pdf
World Health Organization. 2011. Guideline: Use of Multiple Micronutrient Powders for Home Fortification of Foods Consumed by Infants and Children 6–23 Months of Age. WHO Guidelines Approved by the Guidelines Review Committee. Geneva: World Health Organization. http://www.who.int/nutrition/publications/micronutrients/guidelines/guideline_mnp_infants_children/en/
World Health Organization (WHO). 2013. Essential Nutrition Actions: Improving Maternal, Infant, and Young Child Health and Nutrition. Geneva: World Health Organization. . http://apps.who.int/iris/bitstream/10665/84409/1/9789241505550_eng.pdf
Zlotkin, S., S. Newton, A.M. Aimone, I. Azindow, S. Amenga-Etego, K. Tchum, E. Mahama, K.E. Thorpe, and S. Owusu-Agyei. “Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial.” JAMA 2013. Sep 4: 310 (9): 938-47.
Appendices
To read Annex I: Agenda, Annex II: Participant List, and Annex III: Operational Research Topics, please download the PDF above.
